Rhabdo Virus:
Rhabdo viruses are a group of viruses that have wide range of hosts, both plant and animals. Among them, the two are interesting; they are Lyssavirus and Vesiculo virus. Vesiculo viral types such as Vesiculo Stomatitis Virus (VSV)) are pathogens of foot and mouth disease of deadly types. The lyssavirus type is Rabies, which causes, if infected through dog bites, called mad dog disease. Rabies was known since 1808, and Louis Pasteur was the first to demonstrate it and in 1880s he succeeded in isolating attenuated viruses and used it as vaccine against patients bitten by mad dogs. Today scientists have developed vaccines for the same virus.
Rabies is a fatal Central Nervous System (CNS) disease responsible for approximately 60,000 annual deaths worldwide, making it the tenth most common lethal infectious disease. The causative agent is a neurotropic virus consisting of non-segmented, negative-stranded RNA contained within a bullet-shaped envelope. Rabies virus (RV) is 1 of 7 serotypes belonging to the genus Lyssavirus and the family Rhabdoviridae. The most common site of RV entry in humans is the skin or mucous membrane, where the virus is delivered into the muscle and subcutaneous tissue through biting, licking or scratching by an RV-infected animal. Disease can be manifested in one of two clinical forms. In the majority of rabies cases, the pathologic manifestation in the CNS is acute encephalomyelitis. This form is known as classic or encephalitic (furious) rabies and comprises 80-85% of rabies cases. It is distinguished by neurotropism, neuroinvasiveness and impaired neuronal functions. The symptoms of classic rabies include hydrophobia, pharyngeal spasms, and hyperactivity leading to paralysis, coma and death. Paralytic rabies is a less common clinical form characterized by the development of prominent and flaccid muscle weakness. Death in both clinical and paralytic rabies ultimately results from neuronal dysfunction due to the dramatically inhibited synthesis of proteins required for maintaining neuronal functions, (Dietzschold et al. 2005), (Dietzschold et al. 2005).(Plotkin 2000), Warrell et al. 2004). (Jackson 2002), (Hankins et al. 2004).
Genera Properties Members
|
Lyssa virua |
Infect vertebrtes and some insects |
Rabies, Makola, Lagos bat viruses |
|
Vesiculo virus |
VSV humans and cattles |
VSV, Cocal virus,Algoas virus |
|
Ephemero virus |
Infects vertebrates, |
Bovine ephemeral fever |
|
Family |
Genus |
Type Species |
Hosts |
|
Rhabdoviridae |
Vesiculovirus |
Vesicular stomatitis Indiana virus |
Vertebrates |
|
Lyssavirus (Greek, 'frenzy') |
Rabies virus |
Vertebrates |
|
|
Ephemerovirus |
Bovine ephemeral fever virus |
Vertebrates |
|
|
Novirhabdovirus |
Infectious haematopoetic necrosis virus |
Vertebrates |
|
|
Cytorhabdovirus |
Lettuce necrotic yellows virus |
Plants |
|
|
Nucleorhabdovirus |
Potato yellow dwarf virus |
Plants |
|
Rabies is an 'ancient' disease, first shown to be of infectious origin in 1808, shown to be of viral etiology by Pasteur in the 1880's (when Pasteur and Koch were developing the germ theory of disease - prior to the firm modern definition of 'viruses' by Beijerinick (1898)). Over a decade, Pasteur carried out the serial passage of Rabies virus in rabbits, and eventually succeeded in isolating an attenuated preparation which was used to treat patients bitten by mad dogs (not without some risks). at least in terms of phenotypes. All types of Rhabdo viruses show certain diagnostic symptoms.
Vesicular Stomatitis virus; Bullet shaped morphology; http://www.mcb.uct.ac.za/ http://www.asknature.org/
![]()
Rabies-Rhabdovirus; http://bhavanajagat.com/
Affected regions of the body; https://virusbook.pbworks.com
Classification:
General features:
- Most of the Rhabdoviruses are covered by host cellular membranes. Vesicular stomatitis virus (VSV), Rabies virus (RV) and others are few examples out of 200 or more viruses belong to this group. VSV can cause mild febrile disease. VSV causes epidemic disease among the cattle population. Rabies viral deaths in western countries are rare but in Asian countries it is rabid. The viral reservoirs are skunks, dogs, cattle (Brazil?) and bats.
- Most of the viruses of this group show similar characteristics features in morphology and replication mechanisms. They are all RNA viruses with negative sense, which means the RNA, though has information in terms of nucleotide sequences, it cannot be translated (why?); this can be due to the ntds sequence is not in frame, the raison is that the (-) RNAs always have anti sense sequences, so protein synthesis cannot be initiated by the ribosomes in cytoplasm (why?).
- The repositories of the viruses are dogs, vampires, foxes, and cats. Skunks and even cattles act as reservoirs, where Rabies is endemic and rampant.
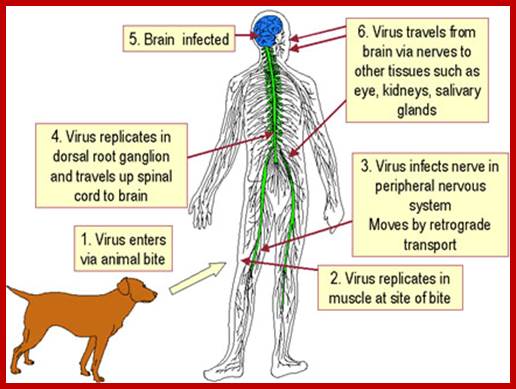
- Initially they multiply in connective tissues and muscle tissues without symptoms and eventually reach peripheral neuronal tissues and then to CNS. In this tissue it causes fatal encephalitis. Initial incubation period, before the disease manifests in humans, is about 3 to 8 months or it may take one year or so, which depends upon the site of infection and size of the inoculum. Severity of the disease can be observed by seeing the patient nearer to death. Fortunately scientists have developed vaccines (attenuated viruses). Human being is the dead end of the viral infection.
Morphology:
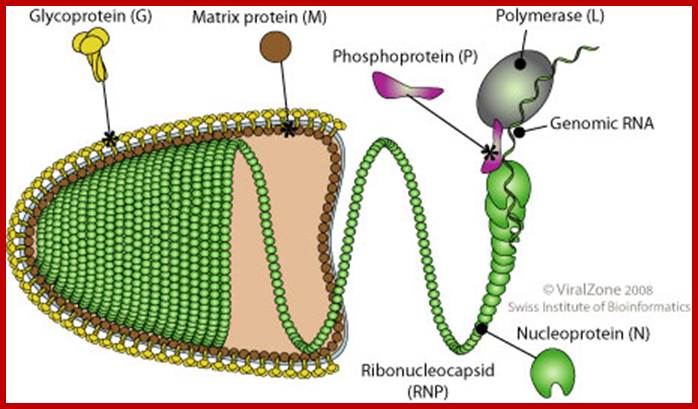
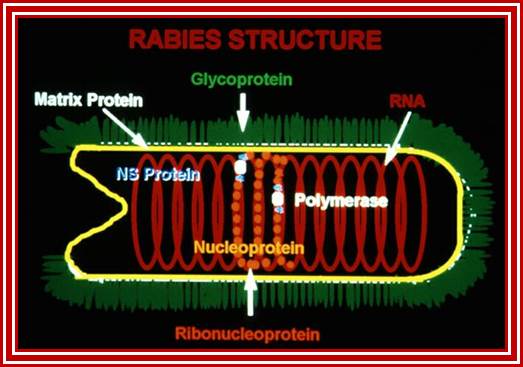
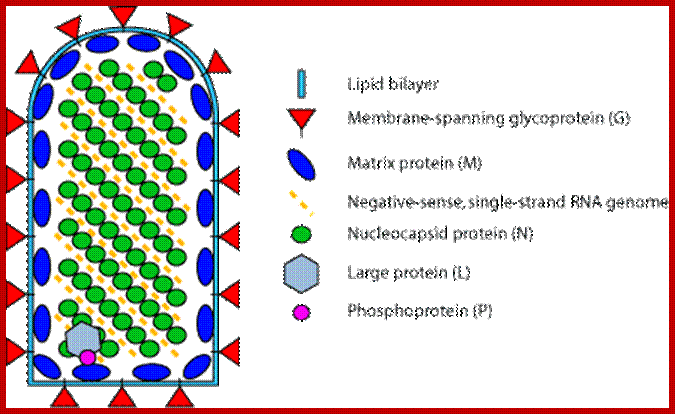
- Rhabdoviruses namely Rabies has a rod shaped or bullet shaped structure of 180x70 nm size. It is enveloped with host membrane, studded with transmembrane glyco- proteins (glycosylation at two sites).
- On the inner surface of the membrane there are a number of matrix proteins, uniformly covered. In the inner space of the virus, a long spirally coiled genomic RNA covered with RNA binding protein called nucleo capsid is present. This thread is like an RNA–chromosome.
- There are approximately 34 -35 coils and each coil has 24 N protein subunits associated within. The RNA-chromosome is associated with N, S and L proteins, which are involved in RNA transcription and replication.
- Most of the negative sense RNA viruses carry their own Replicase enzyme, unlike positive sense RNA viruses, which make their own enzymes upon entry into the cell and translation of its genome.
- The RNA genome, polycistronic, is approximately made-up of 11,153 nucleotides including 5’ UTR and 3’UTR sequences plus IGR +/- (10) 64 = 11216 ntds.

Rabies virions are bullet-shaped with 10-nm spike-like glycoprotein peplomers covering the surface. The ribonucleoprotein is composed of RNA encased in nucleoprotein -(), phosphorylated or phosphoprotein -Illistration of virus, and polymerase -virus.; Another view of bullet shaped Rhabdo virus; http://www.cdc.gov/; http://virology-online.com/
The internal ribonucleoprotein (RNP) core of the rabies virion consists of a negative-sense genome RNA encapsidated by nucleoprotein, polymerase cofactor phosphoprotein, and the virion-associated RNA polymerase. The RNP core is covered in matrix protein and surrounded by a lipid-bilayer envelope.
- The RNA lacks in cap and poly (A) tails, instead they are covered fully including ends with N proteins, and so they are resistant to host cellular exonucleases. The genomic RNA has 59-60 ntds long UTR at 5’ end and a 47-48 ntds at 3’ UTR. The coding region is split into segments for different proteins with intergenic spacers or regions of 16 nucleotides and the sequence is same in all the IGR.
5’I-------I-------------I--------------I---------I---------I---------I--I3’
59 6380 1672 838 822 1333 47 =11162 ntds
L G M P N
Table showing Genes and gene products and functions
|
Gene |
Protein(kd) |
Function |
|
L |
116-190 |
Replicase |
|
G |
65 |
TM glycoprotein |
|
M |
26 |
Matrix protein |
|
NS |
40/46 |
NS-replication |
|
N (p) |
50 |
RNA Replicase |
|
|
|
|
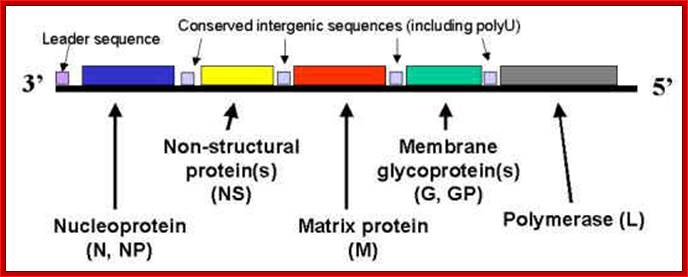
The genomic (-) RNA diagram showing coding region for various genes and in VSV the intergenic spacer with 3’ ACUUUUUUU C/GU UUGUC-5’ sequence that helps in termination of that specific gene and provides the poly (A) additional sequence.
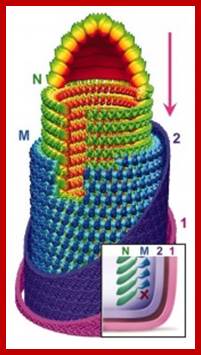
Mononegavirales: Gene order; Helical nucleoprotein 13-20nm wide. http://rhabdoviruses.wordpress.com/
- In between each of the coding regions, there are 16-17 ntd long spacers. The spacers contain consensus sequence of which seven ‘U’s are very important. The sequence in rabies is 3’ ACUUUUUUU C(N)n UUGU G/A. In VSV IGR sequence is 3’ ACUUUUUUU C/GU UUGUC-5’
- Organization of the genomic RNA is like a polycistronic RNA with significant intercistronic spacers.
Replication:
CYTOPLASMIC
- Attachement of the viral G glycoproteins to host receptors mediates Clathrin-mediated endocytosis of the virus into the host cell.
- Fusion of virus membrane with the vesicle membrane; ribonucleocapsid is released into the cytoplasm.
- Sequential transcription , viral mRNAs are capped and polyadenylated by polymerase stuttering in the cytoplasm.
- Replication presumably starts when enough nucleoprotein is present to encapsidate neo-synthetized antigenomes and genomes.
- The ribonucleocapsid binds to the matrix protein and buds via the host ESCRT complexesoccurs at the plasma membrane, releasing new virions. http://viralzone.expasy.org/
- The genomic RNA is assumed to replicate and mature entirely within the cytoplasm, or entirely in the nucleus for it has all the required components for executing the function.
- The genomic RNA when released into the cytoplasm it is already loaded with transcriptional complex of proteins (L PNs).
- Depending upon where the genome replicates and mature, they are basically classified as cyto-viridae and nucleo-viridae. Most of the information for replication and maturation has come from the studies on VSV virus, but now information on Rabies is also available.
- The L(p) NS complex of 250Kd performs different reactions during the course of replication like: 1) Template directed complementary RNA synthesis. 2) Poly adenylation via poly-A signal. 3) Addition of a leader sequence to mRNAs. 4) Addition 7’methyl Guanine to 5’ end of each mRNA segment. 5) Addition of methyl groups.
- In VSV there are two complexes, one act as transcriptional complex and the other acts as replication complex. The transcriptional complex transcribes individual segments and generates positive sense mRNA with 5’cap and 3’Poly (A) tail, however the 5’leader sequence is 47-48 ntds and it is same for all cistronic segmental transcripts are added with 48-49 ntds as UTR. At the other end UTR is 58-59 ntds long.
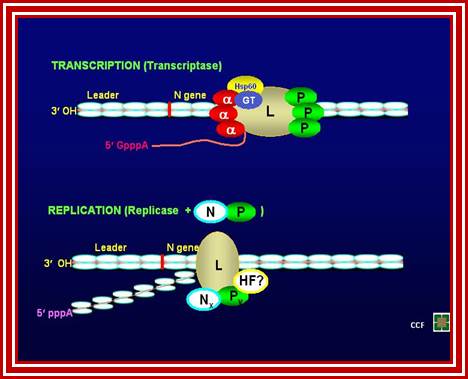
- The transcriptional complex consists of L, P, NS and three of the host proteins EF-1 alpha, HSP 60, Guanylyl transferase.
- The replicase complex consists of L, P and Ns; this is exclusively used for generating full length RNA, both (+) sense transcripts and full length (-) sense RNA.
Recently, by immunoaffinity column chromatography purified two RNA polymerase complexes, the transcriptase and replicase, from VSV-infected baby hamster kidney cells. The transcriptase is a multiprotein complex, containing the virus-encoded RNA polymerase L and P proteins, and two cellular proteins, translation elongation factor-1 a and heat-shock protein 60. In addition, the complex contains a sub molar amount of cellular mRNA cap guanylyltransferase.
The replicase, on the other hand, is a complex containing the viral protein, L, P, and the nucleocapsid (N), but lacking elongation factor-1 a heat shock protein 60, and guanylyltransferase. The transcriptase complex synthesizes capped mRNAs and initiates transcription at the first gene (N) start site, whereas the replicase complex initiates RNA synthesis at the precise 3' end of the genome RNA and synthesizes encapsidated replication products in the presence of the N-P complex.
The two RNA polymerase complexes that differ in their content of virally and host-encoded proteins are separately responsible for transcription and replication of vesicular stomatitis virus genome RNA, as shown in Fig. Understanding the structure and function of the transcriptase and replicase complexes are fundamental to gain insight into the replicative pathways of VSV life cycle.
Mechanism:
· Initially, the L/NS complex binds to 3’UTR region of the N covered genomic RNA and adds RNA nucleotides one by one in 5’>3’ direction, till it generates about 47 ntds long RNA segment. It does not require any primer. All known RNA polymerase transcribe templates with out any primers.
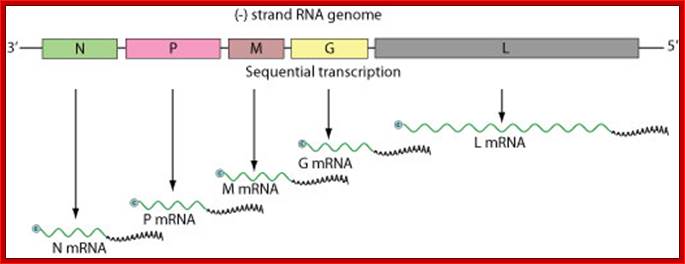
Individual cistrons are transcribed by transcriptase enzyme complex and polyadenylated and also cap is added at 5’end; these RNAs are like eukaryotic mRNAs, but monocistronic; Negative-stranded RNA linear genome, about 11-15 kb in size. Encodes for 5 to six proteins; G-Glycoprotein, NP-Nucleoprotein, P-Phosphoprotein, L-Large protein and M Matrix protein;
Imagefrom: http://expasy.org/viralzone/all_by_species/2.html; http://viralzone.expasy.org/
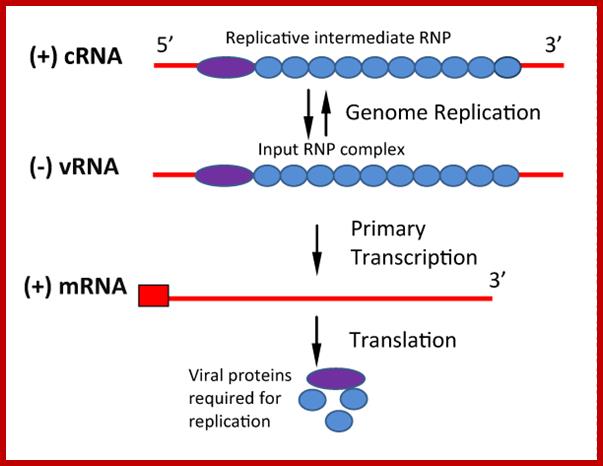
The top figure shows the composition of Transcription complex and replication complex; bottom; Replication of Negative-Sense RNA Viruses: The input genome is a ribonucleoprotein (RNP) complex which cannot be translated. RNA-dependent RNA polymerase is associated with the input genome and transcribes mRNA molecules as the first step of replication. Translation of primary transcripts produces proteins which together with full-length (+) cRNA form RNP replicative intermediate. This then serves as template for new genome synthesis. Both vRNA and cRNA are exactly complementary and encapsidated by nucleocapsid (N) protein, while mRNA is 3' truncated. https://symbiosisonlinepublishing.com
· Then the short segment dissociates and falls off the strand. Whether, this leader segment synthesis is repeated or not clear, but every (+) RNA segment possesses the same 47-ntd long leader sequence with a cap at its 5’ end.
· It is assumed that L/NS complex, (in association with one of the host factors called H) performs not only the addition of the leader and also capping.
· Once the leader sequence dissociates from the enzyme complex, the enzyme continues and transcribes first coding region.
· As the enzyme reaches the end of the coding segment, it encounters a sequence, which provides what is called poly (A) signal, in Rabies-3’ ACUUUUUUU C(N)n UUGU G/A. In VSV IGR sequence is 3’ ACUUUUUUU C/GU UUGUC-5’.
Then the enzyme continues to transcribe the stretch of 7Us, when it reaches the last U, it some how slips back to the first of the seven Us with out leaving the transcript, and moves forward and adds another seven A’s. This way it can add few more seven nucleotide poly (A) blocks, then moves into the next coding segment leaving the transcript free from the enzyme. Individual transcripts are added with their 5’ UTR 47ntds long segment.
· Using this unique mechanism it transcribes all segments and produces five distinct mRNAs. Each of the transcripts is added with a 47-48 ntds long leader sequence generated from 3’ transcript. The transcripts are added with a cap at 5’ end and a poly-A tail at its 3’ end, like any other eukaryotic mRNAs
· The first transcript coded is longest of all the transcripts and last but one is the second longest but it is an important segment. Each of these mRNA segments is translated to produce their respective proteins.
· As more and more of N proteins are produced, they bind to the entire length of RNA. When the RNA is fully covered with the nucleo capsid protein, the L/NS complex that initiates the synthesis of leader sequences, now it doesn’t dissociate from the template and the enzyme continues to produce a full length (+) RNA. Thus many (+) RNA are generated.
· At the same time the mRNA transcripts produced are translated and the products accumulate in the cell. Once the protein products are produced in sufficient quantity, the same enzyme complex, perhaps with one of the host factors, may now switch to full length (+) RNA and initiate (–) RNA from its 3’ end of the (+) RNA.
· Within about 8-10 hrs of infection, thousands of RNA particles are produced and released.
· The mRNA produced for Glycoprotein is translated and transferred into ER and Golgi, where the protein is properly folded and glycosylated at 3 sites containing Asn-X-Serine. Among the three sites one is not efficiently glycosylated. The Matrix and Glyco-proteins produced are transferred to endoplasmic reticulum via signal sequence mechanism. From golgi complex proteins are transferred to the plasma membrane, It is here the viral particle gets it envelope.
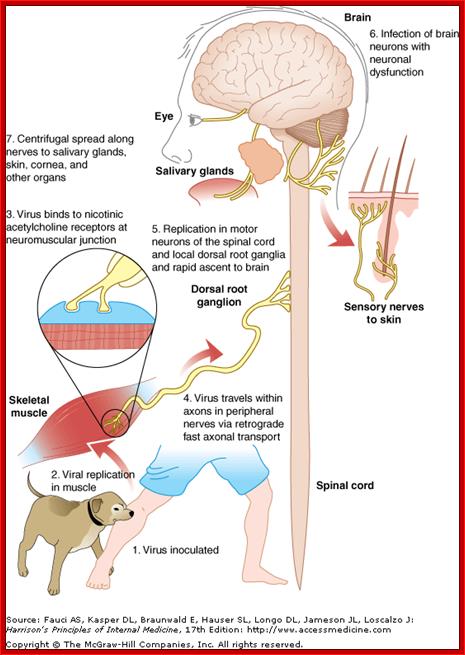
This figure shows systemic passage of virus and affected body parts; incubation period varies from 2weeks to 3 months. After entering viruses spread centripetaly a a rate of 100-400mm/d via fast axonal transport to pinal chord and brain. Once virus enters bran the spread becomes Fster and the symptoms visible. http://dualibra.com/
· As they are processed and transferred to plasma membrane via Vescicular transport mode.
· Full length (-) RNAs, using assembly or packaging sequences bind to plasma membrane where viral proteins are located. It is here the viral particles organize and bud off continuously.
The released viruses enter into blood stream and reach CNS, where they incubate for a long time before they cause full-fledged disease.