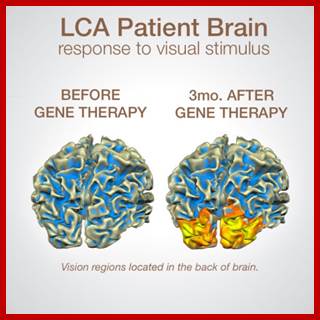
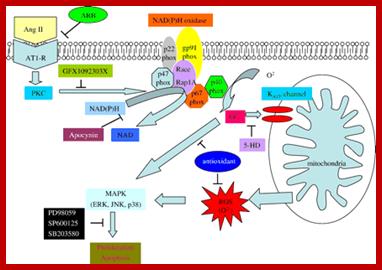
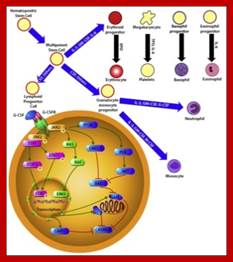
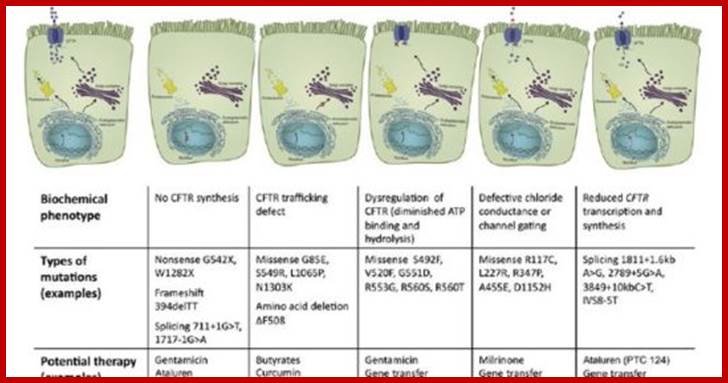
Genetic Engineering:
Application-Animal Biotechnology:
Transgenic animals:
Molecular Medicine:
Ever since the modern human specie arrived on the surface of this planet and started walking around and started living in groups, he collected animals and plants of his liking for his own use. He also reared them and stock piled them under his care. This is the beginning of animal and plant farming. He also observed variation among the same animal and plants cross bred them to develop new varieties, this is yet another technology innovated by him called reproductive breeding, where he used transfer of an entire genome from one form to another. This is nothing short of “Transgenic” methodology. In 20th century one uses techniques where one or two genes are transferred into animal cells and obtain a complete animal called transgenic animals; so also transgenic plants are obtained.
In fact animal cloning experiments started early 1960s and by sixties experimenters were able to get a large number of amphibian clones. The technique was simple; frogs lay eggs outside their body. The eggs were large enough to be seen by naked eye. One can obtain such eggs in large numbers by injecting frogs with reproductive hormones. Enucleation of eggs was a simple technique, which can be perfected with time. Then 2n nucleus sucked from somatic tissues such as intestine, and injected the same into enucleated eggs and prodded the cells to grow; and actually they grew into normal frogs. Several hundreds of such amphibians were grown for experimental purposes. In fact fishes were also used for the same purpose. Some people have perfected culture medium for regrowth of organs such as frog palm or limbs by using special medium containing Vitamin-A.
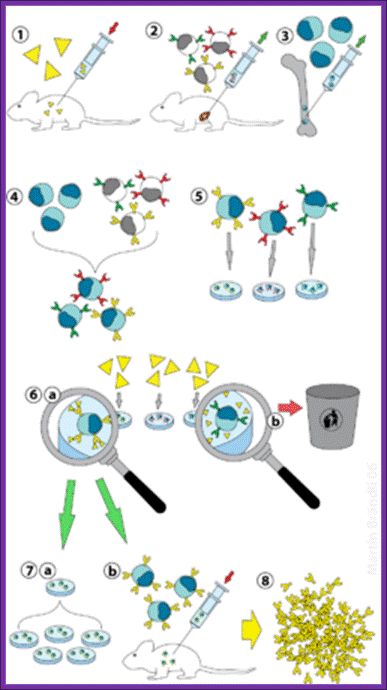
The first transgenic animal produced was from Ralph Brienster, in Lippincott, Univ.of Penn, Philadelphia, in 1980-82 (the author of this website was a witness). They introduced human growth hormone gene into mice eggs.
Donor strain mice were reared and females were injected with pregnant mare serum, or one can use porcine follicle stimulating hormone. After 48 hrs human Gonadotrophin was injected. Hybrid mice are better than inbreed mice?
Such females were mated with selected male studs. Each of the female will produce 18-20 fertilized eggs. After 12 hrs of post coitum, oviducts were taken out and the eggs squeezed out into specific nutrient M16 media.
M16 Media:
NaCl,
KCl,
KH2PO4,
MgSO4,
Glucose,
NaHCO3,
Na Pyruvate,
CaCl2,
HEPES, pH 7.5,
Phenol Red.

http://bsoha.com/

Microinjection-http://www.en.wikipedia.org
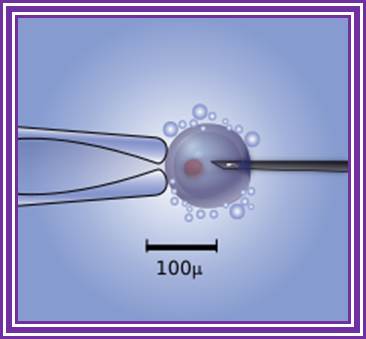
Diagram of the intracytoplasmic sperm injection of a human egg. Micromanipulator on the left holds egg in position while microinjector on the right delivers a single sperm cell. http://www.wikipedia.org

Eggs can be injected with Constructed Recombinant DNA; http://www.hwdsb.onca: www.biotecharticles.com
Gene gun ued to bombard DNA into plant cells
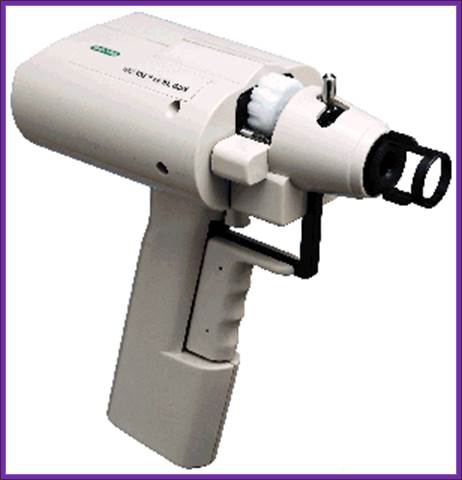
The instrument used for microinjection of DNA into the egg; http://www.bio.davidson.edu/

Natural News Blog; http ; //naturalnewsblog.blogspot.com/
Wash eggs in M16 media and then they are transferred to M2 media micro drops.
Use powerful dissecting microscope equipped with microinjecting facility having very thin and narrow glass tips which can hold only few μl of liquid.
Inject linearized plasmid DNA containing Growth hormone gene into the nucleus of the egg.
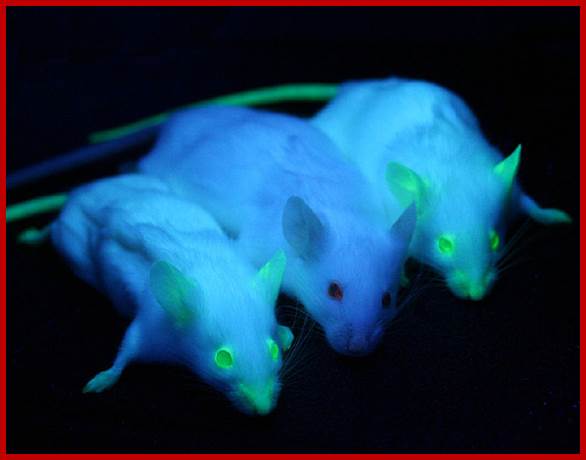
Trangenic Mices with flouescent gene products expressed; http://www.whatisbiotechnology.org/
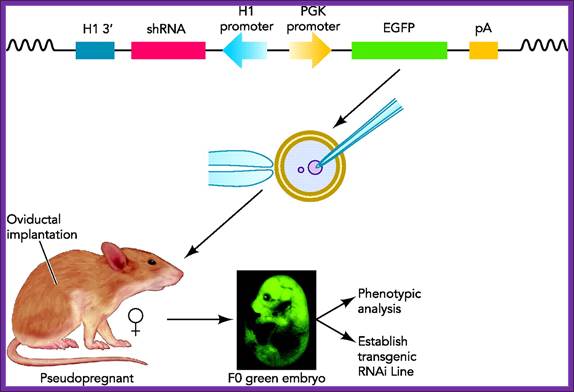
Transgenic RNAi not only allows the study of biological processes not present in cultured cells but also offers chronic therapeutic potentials. In this review, we will summarize the developments in the generation of transgenic RNAi mice.
Transgenic RNA Interference in Mice; http://physiologyonline.physiology.org/
The amount injected is about 2picoliters containing at least 200 copies.
Prepare a set of mice by injecting hormones and mated with vesectomiced males when the females are in estrous stage or what is called animal in “Heat”. These animals behave or rather feel as pseudo pregnant and act as foster mothers.
All these operations have to be done in sterile condition and the job requires skill and deft hands. Under anesthetic conditions these females are operated and the oviduct and uterus is pulled out. Then eggs injected with a gene are transferred into the uterus via infundibulum. Stitch the animal and inject with antibiotics (heavy dose).
In about 19-22 days pups will be delivered.
When they are 10 to 15 days old one can peal off tail skin, for it grows back, and homogenize and isolate DNA and do southern blot, or isolate RNA and do northern blot or isolate proteins and do western blot and find out whether or not the injected genes are integrated and expressed their gene products.


Dr. Ralph Brienster produced first transgenic mices, in the lab (1980-81), Univ. Penn, Philadelphia;
I, Dr. G.R.kantharaj, the author of this website a witness when these mice were born in the Univ. Penn, downtown campus. Dr. Ralph Brienster is great friend of mine https://www.avma.org
Animal Cloning:
Transgenic animals can also be obtained by another method where blastocysts derived stem cells are transfected with the desirable gene placed under specific promoter to express under either induced condition or expressed in tissue specific manner. They are selected against an antibiotic.
They are further amplified and the same are transfused into freshly isolated blastocysts, which are then transplanted into uterus for further development. Many mice cell lines have been created in this manner. One such cell line is used for gene knock out experiment.
Molly and Polly 1977, were obtained as transgenic animals with the gene Factor-IX under the promoter beta lactoglobin for it expression in the mammary tissue during milking period, they used fibroblast cells. A thirty day old fetal cell was used to produce “Transgenic Bull” in 1997 by ABC-Global at Wisconsin. In Honolulu scientists obtained cow.
Cloning word has larger implications in terms of technology, such as DNA cloning, Reproductive Animal cloning and therapeutical cloning. DNA cloning involves cut the DNA and select a specific sequence and ligate it to a vector. Animal cloning involves taking 2n fertilized egg, remove the nucleus and fuse with 2n embryonic cells or adult cells. Therapeutical cloning uses blastocysts or stem cells from different tissues and regenerate the tissue or the organ for transplantation.
Animal cloning was new technique for it was already achieved in 1960s; frogs were the first to be cloned animals. In later years scientists have shown new techniques employed in developing animal cloning. In fact any cell having the full complement of genome is endowed with potentiality to regrow into a complete animal if the cells are provided with proper media and the required vitamins and hormones and stimulus. This is what is called as totipotency of cells. This phenomenon is easily demonstrated plants, where the plants are grown from one or two nucleated cells. This technique of Micropropogation is now employed in agriculture and horticulture.
Professor Sir Ian Wilmut, Edinburgh, the man that led the team in the creation of Dolly the Sheep, She was the most celebrated animal that was obtained, in 1996-97, by modified cloning technique was a sheep called Dolly named after popular singer Dolly Parteon. British scientists (Keith Campbell) from Rosalyn institute had field days of glory and rejoiced in spite of some diehard critics. Dolly started her life, in a test tube, as with all other cloned animals lived. Once normal development was confirmed at 6 days, the embryo, that was eventually to become Dolly, was transferred into a surrogate mother. British scientists (Keith Campbell) from Rosalyn institute countered the knighthood awarded to Sir Ian Wilmut. When Sir Ian was asked directly he replied “I did not create Dolly" was true, Sir Ian replied "yes". He also said that Professor Campbell deserves 66 per cent of the credit for Dolly, although Sir Ian insisted on putting his own name at the beginning of the scientific paper published in the journal Nature in February 1997.
Dolly was born on 5th July, 1996 died at the age of six.
Pregnancy was confirmed by an ultrasound scan at about 45 days of gestation and the pregnancy was monitored closely for the remaining 100 days. The pregnancy went without a problem and Dolly was born on the 5th July 1996. Unlike many cloned animals, which often have neonatal problems at birth, Dolly was a normal vigorous lamb and was standing and sucking unaided within minutes. The animal technicians were aware that this was an important lamb and critical to the research team that had produced her but they were completely unaware of the impact she would finally have. Another scientist to be honored is Debby Reynolds, the UK government's former chief veterinary officer.

Cloning an Epic Historical Mammoth; Animals Whose Genes Have Been Tinkered with by scientists; http://www.iceflux.com/;http://xfinity.comcast.net/
Americans were not far behind for they have also achieved animal cloning especially monkeys. They used different techniques.
Fusing enucleated egg cell with cultured udder epithelial cell from six-year-old female sheep at Go stage, with an electric shock produced dolly. They have made 227 attempts before they were able to get this cloned sheep. Dolly has the distinction of becoming a mother. There are claims that they have used fetal stem cells
There were some claims that the Dolly was obtained from fetal cells. Unfortunately, after all these years of glory, Dolly was believed to be suffered from arthritis. The disease Arthritis may be due to other reasons, not because it is a cloned animal. It has died but left a progeny. Dolly's Family

Dolly and her first lamb, Bonnie, born April 13th 1998 and fathered naturally by David, a welsh mountain Ram; Father of Dolly- awarded knighthood ; Dolly and Bonny; http://www.coldmeadow.com/
In an attempt to allow Dolly to have as normal life as possible, it was decided that she should be allowed to breed.
A small welsh mountain ram was selected as her mate and between them they successfully produced 6 lambs. Their first, Bonny, was born in the spring of 1998. Twins followed the next year and triplets the year after that.
Dolly was made immortalized by duplicating in the wax. www.roslin.ed.ac.uk

Goat /sheep called Dolly? http://www.slideplayer.com/slide
Monkeys were developed by growing an embryo to 8-16 cells and then the cells were separated and grown individually into embryos, which were then implanted into pseudo pregnant mother.
Human cloning was attempted by culturing embryos to 8-cell stage under in vitro conditions and for apparent reasons the embryonic cells are frozen for posterity. Who knows that human clones are not produced???
The clonal sheep Meg, Morgan and Tracy were obtained from embryonic cells. Tracy has genetically manipulated to produce sodium channel proteins involved in cystic fibrosis.
Honolulu people in 1997 also obtained a clone by implanting 2n nucleus into an enucleated egg of cumulina mice. Since then many animals have been obtained as clonal animals. In Japan many cows have been developed as clones. Scientists have attempted to produce pig clones without surface antigens belonging to MHC class.
People have claimed that they have cloned humans and their birth has been heralded all over the world, but no genetic proof for the said claims. Cloning of human beings does not require great technological protocols; in fact with the existing methodologies one can do this in any labs, which have the facility for in human in vitro fertilization and implantation.
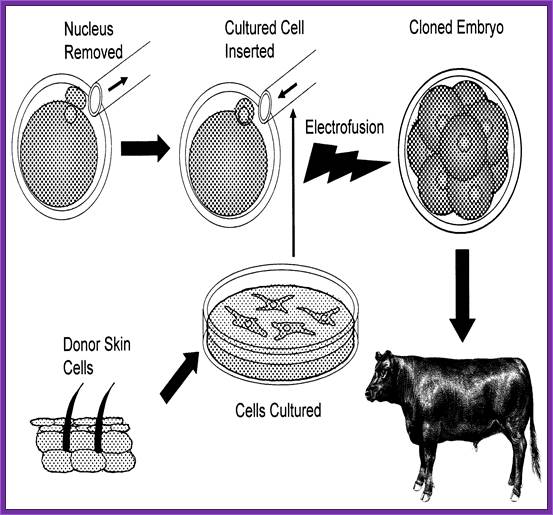
The diagram shows how to develop reconstructed embryo clone;
Robert A. Godke, Richard S. Denniston and Brett Reggio http://www.lsuagcenter.com/

Transgenic Bull Double Muscled Bull Belgian Blue ? https://en.wikipedia.org/
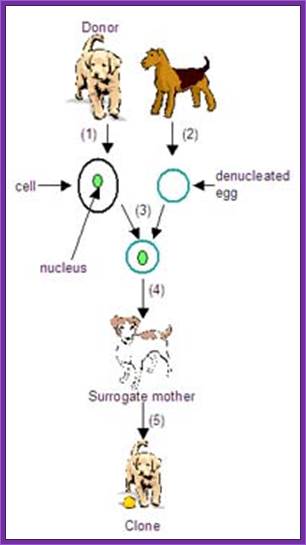
Cloning of a Dog
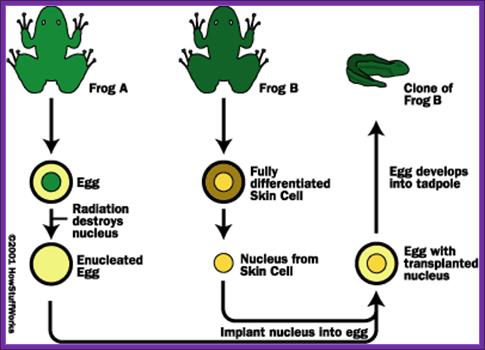
Cloning of Frogs

Ken (left) and Henry were created using DNA plucked from a skin cell of Melvin, the beloved pet of Paula and Phillip Dupont of Lafayette, La.;
Cloned Dogs, http://www.npr.org/

Fluorescent Cats obtained through Genetic Engineering- green and red- transgenic animals- from South Korea. http://news.softpedia.com/
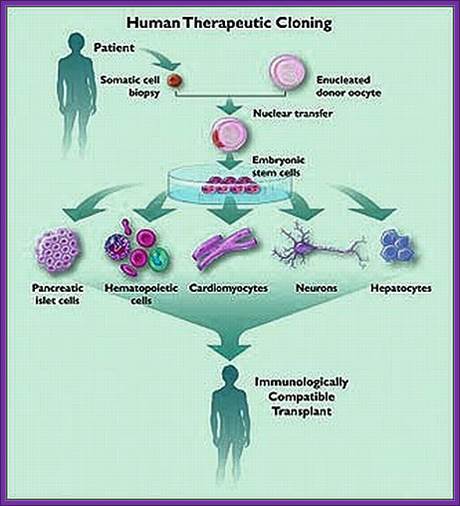
Human cells can be grown and differentiated into specific cell types, then they are implanted. No problem with graft rejection. http://therapeuticcloningprocessandethics.blogspot.in/
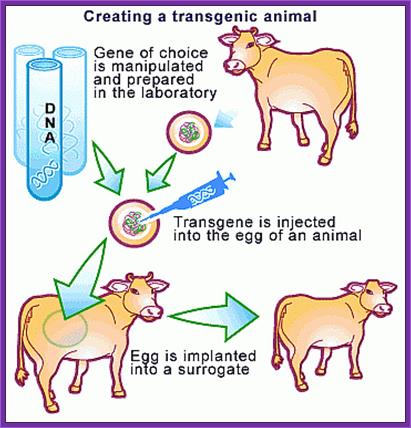
Macdonald farming-Transgenic Cows. https://www.scq.ubc.ca
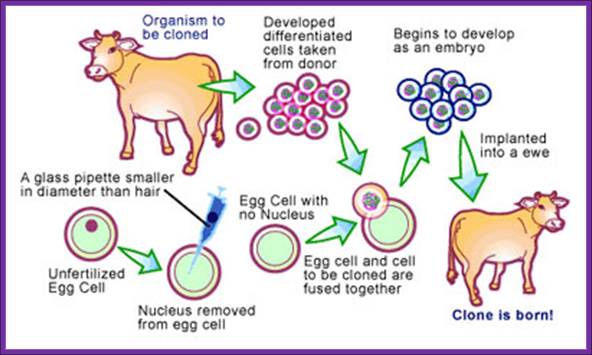
The New Macdonald Pharm; Animal cloning: Cows; Australian scientists have claimed a world first, cloning a cow using a new technique that produces a healthier embryo. https://www.scq.ubc.ca

Genetically modified goats and chickens to produce drugs for humans. But hold on. http://www.salon.com/


Goat n Dog; Dog n Eagle; https://animal-mix.blogspot.in/2007
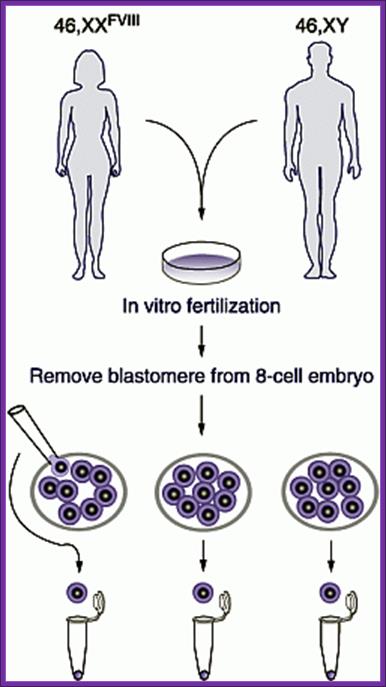
Each blastocyte cell can be implanted in the uterus and grow the embryo.
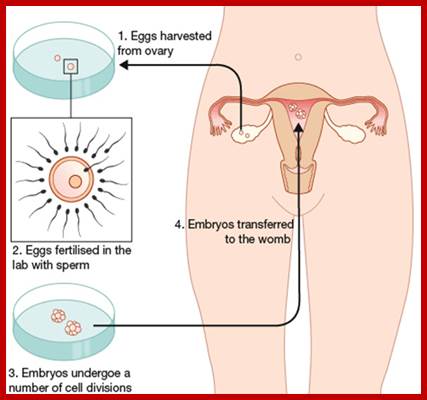
IVF-In vitro fertilization; https://gullalaii.wordpress.com/

In-Vitro fertilization; http://dphx.org/respect-life
Gene ‘Knock Out’ pig ; First knockout pig: alpha 1, 3 galactosyltransferase; http://arbg.missouri.edu/

Lovely cloned cat; but the first cat cloned was from South Korea. www.slideshare.net

Cloned cow; http://blogs.discovermagazine.com/

Animal Farm Catch-;Cloned cow-calf- http://www.ibtimes.com/

Fourth Generation of Pig clones born Cloned piglets, Oh my god! they are so lovely and loving; http://pinktentacle.com/2007/08/fourth-generation-pig-clones-born/

I love these kids; are they cloned Kids?
13. Hybridoma Technology:
This ingenious technique was developed around 1975-1976. The production of monoclonal antibodies was invented by Cesar Milstein and Georges J. F. Köhler in 1975. Monoclonal antibodies were once considered as magic bullets that can target a cell, a tissue, molecule and or an infectious agent.
Polyclonal antibodies are produced by B-lymphocytes. When a specific antigen comes in contact with specific receptors of B-lymphocytes, the genes within the cells get activated and start producing antibodies against the specific epitope that are presented to the lymphocytes. Epitopes are the antigenic determinants of antigen.
Antigens consists of different motifs, different domains derived from amino acid sequences which generate different secondary structural features. Each of these specific domains is a distinct motif. They are recognized by different B. Cells, for they carry specific receptors for a specific epitopes. If an antigen has twenty different epitopes twenty different species of B-cells get activated and twenty different antibodies are produced in one to one manner. Production of antibodies to each of the epitopes is not equal, some elicit weak response and some elicit strong response. Thus the antibodies produced are Polyclonal in nature.
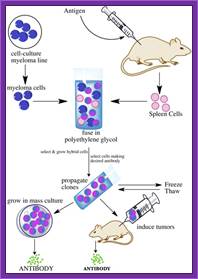
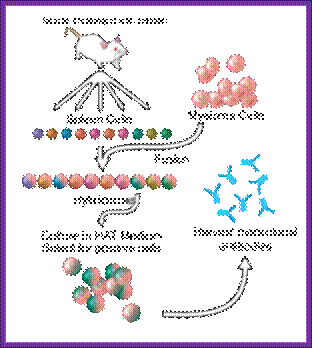
Summary of Hybridoma Method of mAb production; http://php.med.unsw.edu.au/
(1) Immunization of a mouse; (2) Isolation of B cells from the spleen; (3) Cultivation of myeloma cells; (4) Fusion of myeloma and B cells; (5) Separation of cell lines; (6) Screening of suitable cell lines ;(7) in vitro (a) or in vivo (b) multiplication; (8) Harvesting; https://en.wikipedia.org
http://www.getdomainvids.com/
Many a time’s Polyclonal antibodies are not useful for specific purpose. Yet Polyclonal antibodies are used on large scale for it is easy to obtain such antibodies and cost of production is relatively low.
Monoclonal antibodies are for a specific epitope. Under certain situation one wants to target only one of the epitopes and in such circumstances monoclonal antibodies are very useful; that is why they are called as magic bullets. Production of monoclonal antibodies is possible by Hybridoma technology.
B-Lymphocytes:
B-Lymphocytes are capable of producing antibodies in response to specific antigens.
These cells cannot grow and multiply in an in vitro culture medium. They can survive for some time and use salvage pathway if they are grown in the presence of Hypoxanthine, Aminopterin and Thymidine (HAT) medium. Cells cannot multiply in culture conditions. These cells have HGPRT and Thymidine kinase genes.
They can use hypoxanthine for purine synthesis even though Aminopterin blocks DHFR, they have salvage pathway. The reason is the cells have enzymes called Hypoxanthine Guanosyl Phosphor Ribosyl Transferase (HGPRT).
HGPRT defective Myeloma cells:
They are incapable of producing antibodies. They are the transformed B-cells.
They have the ability to multiply under culture conditions by cell division.
Mutant cell lines, cannot utilize Hypoxanthine and Thymine in the presence of Aminopterin for they lacking in HGPRT and Thymidine kinase (TK).
Protocol: Inject the required antigen subcutaneously to a rabbit skin. After three such injections (second and third are called boosters), sacrifice the animal and take out spleen tissue and isolate B-lymphocytes. These lymphocytes are activated to the injected antigen. The cells are capable of producing Polyclonal antibodies.
B-lymphocytes are then fused to HGPRT (-) Myeloma cells. The hybrid cells now can grow and multiply in culture medium even in the presence of Aminopterin. At the same time they also secrete respective antibodies to specific epitopes. Such cells are called Hybridoma cells.

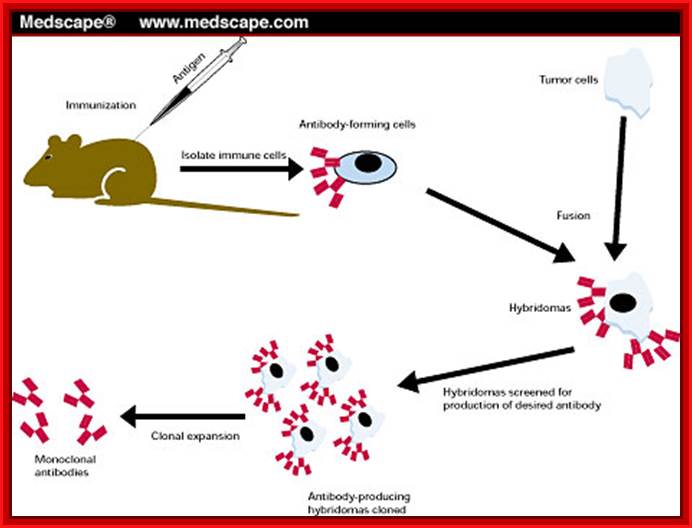
A general representation of the hybridoma method used to produce monoclonal antibodies; César Milstein and Georges J. F. Köhler in 1975. The term hybridoma was coined by Leonard Herzenberg during his sabbatical in César Milstein's laboratory in 1976–1977 ;
Hybridoma Technology; Protocol. https://en.wikipedia.org
Such cells are diluted and dispensed into multi titer plates in such a way that each well contains one such Hybridoma cell. Such cell lines divide and redivide, grow and produce antibodies to one specific epitope.
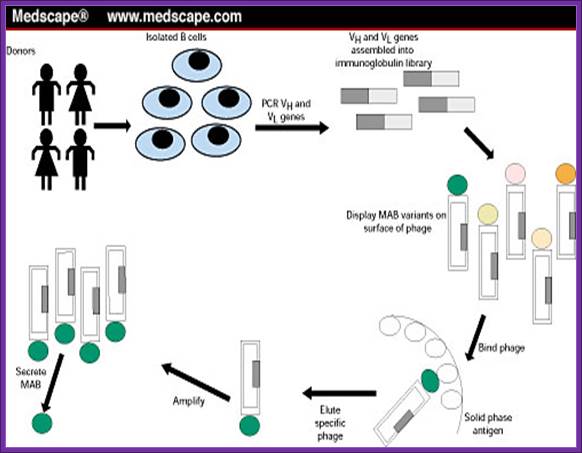
Medscape.com
Using a specific epitope from the antigen one can screen cells from each well and pick whatever clone from the titer plate one desires. The same cell line can be propagated and maintained.
Monoclonal antibodies are very useful tools in research.
One can trace the position of an antigen.
Scarce proteins can be purified.
Used for disease diagnosis.
Such cells can be used to treat patients infected with a particular infectious agent.
It is possible to target a specific malignant tumor cell or cells and kill them by directing T-killer cells to them.
21. Molecular diagnostics:
Infectious diseases are generally identified by symptoms, culturing and microscopic examination. And many of the investigations are also done by biochemical analysis. These methods are still in vogue. But certain infectious diseases take a long time to manifest the symptoms and often-early diagnosis leads to miss interpretation. Example malarial infection takes a month or so to fully blown manifestation. On the contrary HIV infection may take a year or so. By the time theses are diagnosed, treatment will be delayed and it can be disastrous. Of late molecular diagnostic tools have been evolved. One such technique is ELISA (Enzyme Linked Immuno Sorbent) Assay) technique has been developed, either using Polyclonal antibodies or monoclonal antibodies. This is certainly very effective, but not all diseases can be identified by this technique and expensive. Yet ELISA is a powerful and highly sensitive diagnostic method, which is used in medicine, research and industry. Definitely a monoclonal antibody technique is very very costly. Along with ELISA protocols, another technique called PCR has been developed to identify not only diagnosis of infectious agents but also to detect any suspected genetic diseases. For example HIV infection can be positively diagnosed by PCR method. Combined with ELISA the diagnosis is 100% efficient.
Antibodies as diagnostic tools:
Antibodies have to be raised for every antigen bearing infectious agent. Most of the antibodies are polyclonal. Some times Polyclonal antibodies cannot distinguish between pathogenic and nonpathogenic germs. Hence people started using monoclonal antibodies, which was found be costly and not within the reach of common folks. But now it is possible and in reach of common man.
A List of antibodies employed in diagnosis (just few examples):
IgGs for Polypeptide hormones:
Chorionic gonadotrophin.
Growth hormones.
Leutinizing hormones.
Follicle stimulating hormone.
Thyroid stimulating hormone.
Prolactin.
Cytokines:
Interleukin 1-8.
Colony stimulating factor.
Tumor markers:
Carcinoma embryonic antigen,
Prostrate specific antigen,
Interlekin 2 receptor,
Epidermal growth factor.
Drug monitoring:
Theophylline,
Gentamycin,
Cyclosporin.
Others:
Thyroxine,
Vit-B12,
Ferritin,
Fibrin degrading factor,
Few examples IgGs against disease causing organisms:
Chlamydia,
Herpes simplex,
Rubella,
Hepatitis-B,
Hepatitis A,
Hepatitis-C,
Schigella,
Mycobacterium,
HIV,
HPTLV,
Cholera toxins,
Botulins,
Polio,
Nematodes,
Malarial antigens,
A large number of viral antigens and bacterial antigens and protozoan surface antigens have been used for developing diagnostic kits. The above list is to give an idea what are few diagnostic IgGs.
In recent years instead of preparing IgGs for every antigen, scientists have used total human lymphocytes and prepared a composite but combinatorial cDNA library for both Heavy chains and light chains. Lambda DNA has been used to prepare such library. This method can generate 10^6 to 10^8 clones, each distinct from the other. Proteins expressed can be screened with diagnostic antigens. Now days the antibodies are generated using genetic manipulation in such a way the antibodies when injected to human, they don’t elicit immune response and such antibodies are called humanized antibodies.
DNA as the diagnostic material:
This has become a sine quo non technology when everything has failed. This technique has undergone lot modification and sophistication. The method is simple and fast. This technique can be used to detect not only disease causing pathogens but also to detect parental pattern, if you are in doubt.
Genetic defects.
Most important requirement for PCR based diagnostic methods is knowledge of the pathogen’s DNA and its sequence. From the sequence one can generate a set of primers for 5’ and 3’ ends. Known primers can employed to identify. The source of material required is tissue sample, fecal material, urine, blood sample, throat smears or throat wash, a hair follicle. The amount DNA required can be as small as few picograms. In order to combat disease all over the world, WHO has a program to develop 300- 500 diagnostic kits.
Diagnosis of Malarial parasites:
Full-blown disease manifestation takes 15 days to one month. Malarial disease is caused by plasmodium falciparum. Elisa is effectively used, but the parasite often camouflages by withdrawing its surface antigen and projecting another antigen. Here the probe used is highly repetitive DNA sequences. Such sequences when used they are distinguishable from P.falciform with other P.vivax, P.cyanomoglia and other related parasites. Blood sample is more than enough diagnosis of the disease.
Similar highly repetitive sequences have been used to detect disease-causing parasite such as Trypanosome cruzi (chagas disease). Amplification of the said DNA using specific primers it is possible identify this disease with accuracy and other related forms are not detected. It is very specific. This disease has devastated South American population.
Diagnosis of DMD:
Duchene’s muscular dystrophy sex linked disease, generally appears at greater frequency in male children than the female for the females are the carriers. Here the diseased children suffer from muscular wastage for the surface protein Dystrophin is non functional. This protein is found in the myelin sheath at the inner surface covering the axons. The DMD gene is located in the XP21 arm.
The fully processed DMD mRNA is 14 000 nucleotides long. The pre mRNA consists of `60 Exons. The whole gene extends to an area of 20000kbp. The protein has a molecular mass of 600 KD; perhaps it is one of the largest proteins known in eukaryotic organisms. In fact the largest protein is ‘Titin’.
When the DMD gene is cut with Taq-I enzyme, it generates seven fragments. Sites for the Taq-I enzyme is found in few introns. Scientists have developed primers for each of the fragments and the same can be used for PCR amplification. Control DNA generates 7 fragments of the size 10KB, 7.8 kbp, 4kbp, 3.8kbp, 3 kbp, 1.8kbp and 0.6 kbp and they can be discerned on an Agarose gel. If a suspected patient’s DNA is used one will find some segments of the Gene are missing, using multiplex PCR it is possible to diagnose the disease in a short time; and that too with certainty.
The same technique can be employed in identifying phenyl ketoneuria, Sickle cell anemia, Hemophilia, Cystic fibrosis and others.
Diagnosis of Hemophilia:
Hemophilia polymorphism is due to change in restriction sites such as Xba-I and Bcl-I. This is due to mutation in the sequence of these sites. Hemophilia X factor has 26 Exons. Abnormality was found in the 18 Th introns. A pair of primers were developed for amplifying a 142 bp long DNA involving the introns. After PCR amplification of the segment it was cut with Bcl-I and separated on a gel. A patient, whether he or she is heterozygous and homozygous can be discerned from the gel pattern.
|
Control (Homozygous) |
Control (Heterozygous) |
Patient-1 |
Patient-2 |
|
|
|
----- |
------ |
----- |
(-) Bcl-I 142 bp |
|
------ |
----- |
------ |
|
(+) Bcl-I 99bp |
|
------ |
----- |
----- |
|
(+) Bcl-I 43bp |
|
|
|
|
|
|
Multiplex PCR is used to detect mutations in b-Globin genes. Primers are used are fluorescent labeled. Thereby they were able to detect two mutations in b-Globin genes, ands one mutation in alpha Globin gene.
Human Genetic Diseases:
It has been estimated that human genetic disorder and their manifestations in the form of diseases is about 3000 and odd. The diseases can be autosomal or X-linked. Mutations can be dominant, negative dominant, recessive, can be incomplete penetrance, may show variability in expression, show heterogeneity, and or exist in alternate forms of the same gene. Genes are pleotropic.
Therapeutical Agents.
Any chemical or biochemical compound designed to combat disease fall into the category called Therapeutical agent. Since the days of Edward Jenner, early 200 years ago, scientists are in hot pursuit of discovering drugs against pathogen. Vaccination against pathogens worldwide is the most effective preventive therapeutical agent next only to antibiotics. Discovery of Antibiotics is a great event in the history of mankind, but over use and abuse of antibiotics resulted in the pathogens have become or becoming resistance to drugs. It is constant and eve going struggle against pathogens and drug discoverers, who dominates that is the question is like survival of the fittest.
Vaccines:
Edward Jenner was an incredible country doctor, using folklore knowledge; he injected liquid from the cowpox pustule into an eight-year-old boy called James Philips. It was a history unsurpassed of any discovery the mankind knows in centuries.
Most of the infectious agents infection spread of some of them in epidemic, rarely in pandemic proportions can be prevented by vaccination. Any immunogenic injected into human body, elicits immune response against that antigen. The efficiency of response depends upon the epitopes found on the presenting surface of the antigen.
According to ‘WHO’ the number of communicable disease against vaccine to be developed by 2000 was 400. And the target now is much more. Conventionally vaccines were prepared by using heat killed or formal in killed or inactivated organisms as inoculants. Though maintenance of them and obtain them as pure forms endowed with some difficulties, it is still the cheapest for these have to be prepared in large quantities for global market.
With the advent of Molecular and gene engineering techniques, recent trend is to develop recombinant vaccines and many such products are made available for large-scale vaccination, ex. Vaccination against Hepatitis-B virus. The modern methods have been developed to overcome drawbacks of traditional methods.
Methods employed for developing recombinant vaccines:
- Virulent genes of an infectious agent is deleted or made nonfunctional or dysfunctional and such organisms can be used as live vaccines. It has to be made certain that the organism does not regain that gene or function.
- Live nonpathogenic organism can be used as carriers. These organism can genetically manipulated in such a way an antigenic determinant gene is introduced into the organism where it presents the antigen to immune system, thus such organisms can be used as vaccines. But the antigenic determinant used should elicit strong response.
- Develop alternate host or forms for those organisms which cannot be grown and maintained on large scale, ex. Mycobacterium lepri. These have to be grown and sustained in the sub-epidermal tissues of Armadillo, which itself is rare to find in nature. In such cases the target gene has to be cloned into either bacteria or into yeast cells so large-scale preparation are made easy.
- Some times infectious agents do not kill host cells, instead host immune system attacks infected cells and kills them. It is now possible to create targeted and cell specific killer systems so as to destroy the infected cells.
Subunit Vaccine:
Instead of using the whole virus or bacterial cell or pathogenic animal cells, a specific component of infectious organism is used, such as viral capsid protein, surface glycoprotein, cell wall antigenic protein or glycoprotein can be effectively employed for eliciting strong immuno-response. The said genes can be cloned into expression system and the same can be purified and can be used vaccines. Such vaccines are stable, safe and the antigens are precisely defined and free from extra cellular contaminants. But the process is costly and many a times immune response is good as expected, but that can be overcome by certain modifications.
Subunit vaccine against Herpes simplex virus:
The viruses cause deadly sexual diseases not only in males but also in females, where they act as reservoirs and males as transmitters. Great many people belonging to higher social status have succumbed to these viruses and died with remorse. The virus is an enveloped type and the protein used is protein-D (gD) is a glycoprotein; it elicits good immune response per se. The said protein does not cause any disease or any other side effects.
The gene for this protein has been cloned into transfer vectors or episomal vectors and transfected Chinese Hamster Ovarian cell lines (CHO) produced properly folded and glycosylated proteins which are as good as the typical viral protein. When the gene was cloned in secretory mode the proteins are secreted out of the cell into the media.
Foot and Mouth Disease Virus (FMDV):
This virus has the ability spread across the continents and cause very severe damage to live stock especially Cattles and Swine. This is very severe in South America, even Indian animal population also suffers but Indian medicine has taken care of the problem. The medicine is a concocted extract of Curcumin, Garlic, Oscimum, Gingiber offcianale and Leucas aspera leaves and a little bit fresh capsicum. If this given a week or so the disease is cured. However the world over requirement of vaccine against this virus is not less than more than 2 billion doses.
The surface protein that is very antigenic is the coat protein called VP1. This gene has been cloned but the protein per se was found to be very poor antigenically. The virus possesses ~8000ntds long RNA genome and the cloned was expressed as fused protein with MS2 Replicase as a carrier protein. Even this was not effective in eliciting a good response.
Scientist looked into different motifs of the protein and the same were selected and cloned; such as- from C-terminal 141-160=21aa, 151-150=11, 200-213=13aa, from the N-terminal sequences such as 9-24aa, 17-32aa, 25-41. These subunits were used with a carrier protein and all of them were found to be very immunogenically. However fused segments from141-158 to 200-213 was found to be stimulatory but 1000x weaker than killed viruses. But when the subunit from 142-160 was fused, Hb-surface antigen was found it was highly immunogenic.
Live recombinant Vaccines:
Methods employed- one is use nonpathogenic organism and genetically modify so at express the desired antigenic protein in proper perspective. The second alternative is use pathogenic organism and delete the virulent gene responsible for causing the disease and retains its antigenic characters.
Live cholera vaccines:
Live vaccines are believed to be more advantageous than subunit vaccines. Cholera is a deadly disease recurs periodically in tropical countries and kills loot of people. Symptoms are fever, abdominal pain, vomiting, diarrhea, which if not take care of will result in death. In many part of the world it is an endemic disease caused by bacteria called Vibrio cholerae, for on infection it colonizes small intestine, which is the target tissue and the bacteria secretes lot of hexameric enterotoxins.
This is the causative agent consists of subunits-A and the other 5 subunits called B. The sub unit-A has ADP-ribosylation activity and stimulates Adenyl cyclase. The A-subunit has A1 domain, which contains functional domain the other part help in joining A to B. They bind to intestinal mucosal receptor and activate intestinal problem.
First deleting the A1 subunit part of the gene by homologous recombination method, in this process tetracycline gene is introduced in the place of A1 subunit segment has developed recombination live vaccine.
Plasmid containing homologous segments and a tetracycline gene id transferred to bacteria Cholera Vibrio. This leads to recombination and deletes the A1 subunit. But this cannot used for it has tetracycline gene. So the enterotoxin gene was isolated and the A1 subunit deleted and plasmid is created containing the deleted enterotoxin gene with homologous segments at both flanking regions. This plasmid is transferred to bacteria with deleted enterotoxin gene. By recombination method the defective enterotoxin gene incorporated and the Tetracycline gene is removed. Such genetically manipulated bacteria are used as live vaccine. It is incredible feet indeed
HB-sAg vaccine:
HB-Ag gene form hepatitis was cloned into a plasmid contain Vaccinia strong promoter. The flanking region of this construct contained homologous segments of Thymidine Kinase gene. When this plasmid and wild Vaccinia viral DNA is co-infected into desired cell lines, by homologous recombination process the Hb-Sag gene is incorporated in the place of Thymidine kinase. This can be screened by Glycover for the absence of Thymidine kinase. This results in the expression surface antigen of Hepatitis, which can be used for vaccination purpose.
Employing the above method a wide variety of genes have been cloned to produce vaccines, ex. Rabies viral G-protein, Sindbis surface antigen, Influenza Np and HA protein, HSV glycoprotein, Vesicular stomatitis N & G protein and many others and used them for vaccination purpose.
Similarly adenoviruses can be used as live viruses. This achieved by deleting replicative function of viruses and desired gene is cloned under the control of early genes. Living viruses is generated using helper viruses. Such viruses have been created for curing cystic fibrosis, a deadly disease among Greek and Cypriot population, where diseased children die before 20 yrs. When recombinant virus id produced with a cystic fibrosis, channel protein gene with flanking regions such as LTR sequences, on delivery the Gene gets integrated into host cell genome. This virus can be delivered into host by nasal spray.
Genetic Immunization:
A cloned gene construct which is capable of producing an antigenically active protein, when biolistically injected into the ears of a mice, the Gene with its borders (LTR) gets integrated and expressed a protein. The protein now acts as an antigen and animal’s immune system responds and mounts antibody production against the protein. This technique has a promising future. When humans are very young as old as 2-3 years it is possible to inject such cloned DNA into body cells to make the child immune to a given antigen. However continuous production of such antigens with in the body will have repercussion effects and how the body can tolerate such continuous stimulation is another question to be answered. Another problem is the quantity of the antigen produced, there is no regulation. I f the production of antigen is made to be regulated with specific stimulation, probably serves the system good.
Use of antibodies as drugs:
Coryneybacterium diphtheriae causes a severe disease faster and kill the patient fast. It first shows infected symptoms in the throat and tonsils, where it generates exotoxins, which on circulation can severely damage organs, which are distant from the site of infection.
People use passive immunity against such disease causing agents. By raising antibody in horse against such bacteria and then injecting the serum into humans, results in developing passive immunity. However second time injection with horse serum will be so serious; it may give anaphylactic shock and cause death. Yet the use of antibodies against a variety of disease causing agents and curing the disease is prevalent. New methods and new approaches to the problems have been in vogue. Monoclonal antibodies are once considered as magic bullets, but because of immune reactions to animal antibodies it became imperative develop antibodies, which are human compatible, called, humanized antibodies. Though monoclonal antibodies are expensive, they are still used in diagnostics; tissue imaging and some times they are used as immuno-suppressants.
Among lymphocytes, T cells which differentiate in Thymus cells act as immunological helper cells and also effecter cells. They are involved in rejection tissue grafting. If grafting to be successful T-cell mediated immune response to foreign tissue should be suppressed, thus one can save lives of a large number of human beings. Researches developed antibodies against T cell receptors called CD3. The first monoclonal antibody developed against CD3 in mice is called OKT3. When injected it binds to the receptor and prevents full scale mounting of immune response to tissue grafting. This kind of treatment was first approved by United States food and drug administration. Without the approval no one can try to use trial on human beings at least in the US of A.
Such monoclonal antibodies are still in use in hospitals where bacteremia is endemic and kills lot of patients in USA. Monoclonal antibodies were used to suppress proliferation of breast cancer cells by raising antibodies against specific cell surface receptors on tumor cells. When such antibodies were used the cell surface receptors were blocked and growth of tumor has become stagnant. Further more once the growth of tumor cell is halted, some factor should be added to cause apoptosis of cancer cells only.
Monoclonal antibodies (mAb or moAb) are monospecific antibodies: Targeting mAbs to specific protein/glycoprotein sites:
Blood clotting in vessels leading to brain and heart can lead to stroke and heart attack. The blood clot in vessels is called Thrombus; it consists of a network of fibrin, one of the primary blood-clotting agents. A defect in the endothelial layers of blood vessel is the cause. Thrombus kills millions of people all over the world, especially in developed world.
Plasminogen (86KD), a precursor protein for plasmin, is a serine protease. The precursor Plasminogen is activated by Plasminogen activating factor (70KD). Once activated the plasmin acts on fibrinogen and fibrin and degrades the clot.
Raise antibodies to human fibrin and conjugate it with Plasminogen activating factor in such a way the enzyme’s active site is not disturbed. When such conjugated composite factor is injected, it homes to the site wherever fibrin is present. As most of the clots contain fibrin, the plasmin a serine protease acts and hydrolyses these proteins using serine sites in the protein. Plasminogen is also activated by Urokinase (54KD). Even Streptokinase takes part in dissolving blood clots. It is important to note that using monoclonal antibodies are used to deliver the required enzyme to specific site in the body. This has great implications in curing several diseases. While treating patients additional factors are also added with t-PA to inhibit t-PA activity, other wise it can lead to internal bleeding.
A general representation of the methods used to produce monoclonal antibodies; http://en.wikipedia.org/
https://www.google.co.in
Monoclonal antibodies for cancer. ADEPT, antibody directed enzyme prodrug therapy; ADCC, antibody dependent cell-mediated cytotoxicity; CDC, complement dependent cytotoxicity; MAb, monoclonal antibody; scFv, single-chain Fv fragment (WIKIPEDIA).
The first FDA-approved therapeutic monoclonal antibody was a murine IgG2a CD3 specific transplant rejection drug, OKT3 (also called muromonab), in 1986. This drug found use in solid organ transplant recipients who became steroid resistant. Hundreds of therapies are undergoing clinical trials. Most are concerned with immunological and oncological targets (Wikipedia).
|
Antibody |
Brand name |
Approval date |
Type |
Target |
Indication |
|
ReoPro |
1994 |
chimeric |
inhibition of glycoprotein IIb/IIIa |
||
|
Humira |
2002 |
human |
inhibition of TNF-α signaling |
Several auto-immune disorders |
|
|
Campath |
2001 |
humanized |
|||
|
Simulect |
1998 |
chimeric |
|||
|
Benlysta |
2011 |
human |
|||
|
Avastin |
2004 |
humanized |
Vascular endothelial growth factor (VEGF) |
Colorectal cancer, Age related macular degeneration (off-label) |
|
|
Adcetris |
2011 |
Chimeric |
|||
|
Ilaris |
2009 |
Human |
|||
|
Erbitux |
2004 |
chimeric |
|||
|
Cimzia |
2008 |
humanized |
inhibition of TNF-α signaling |
||
|
Zenapax |
1997 |
humanized |
Transplant rejection |
||
|
Prolia , Xgeva |
2010 |
Human |
|||
|
Soliris |
2007 |
humanized |
Complement system protein C5 |
||
|
Raptiva |
2002 |
humanized |
|||
|
Mylotarg |
2000 |
humanized |
|||
|
Simponi |
2009 |
Human |
Rheumatoid arthritis, Psoriatic arthritis, and Ankylosing spondylitis |
||
|
Zevalin |
2002 |
murine |
Non-Hodgkin lymphoma (with yttrium-90 or indium-111) |
||
|
Remicade |
1998 |
chimeric |
inhibition of TNF-α signaling |
Several autoimmune disorders |
|
|
Yervoy |
2011 |
Human |
|||
|
Orthoclone OKT3 |
1986 |
murine |
Transplant rejection |
||
|
Tysabri |
2006 |
humanized |
alpha-4 (α4) integrin, |
||
|
Arzerra |
2009 |
Human |
|||
|
Xolair |
2004 |
humanized |
immunoglobulin E (IgE) |
||
|
Synagis |
1998 |
humanized |
an epitope of the RSV F protein |
||
|
Vectibix |
2006 |
human |
epidermal growth factor receptor |
||
|
Lucentis |
2006 |
humanized |
Vascular endothelial growth factor A (VEGF-A) |
||
|
Rituxan, Mabthera |
1997 |
chimeric |
|||
|
Actemra and RoActemra |
2010 |
Humanised |
|||
|
Bexxar |
2003 |
murine |
Non-Hodgkin lymphoma |
||
|
Herceptin |
1998 |
humanized |
|||
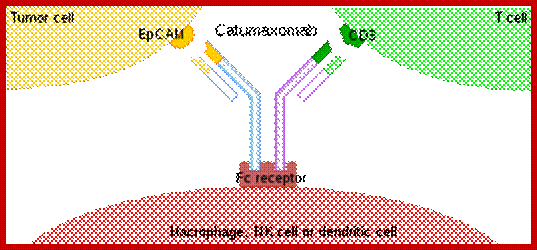
Recently, the bispecific antibodies, a novel class of therapeutic antibodies, have yielded promising results in clinical trials. In April 2009, the bispecific antibody catumaxomab was approved in the European Union.
Abzymes:
By genetic manipulations it is possible to convert antigen-binding site into an active site of the enzyme’. This product has the ability to recognize the specific substrate as well as to perform enzyme activity.
Use of mABs in treatment has created problems for they themselves act as antigens, so they have modified the IgG in such way they act and behave like human antibodies (humanized). In most of the cases monoclonal antibodies were obtained from mice, and mice antibodies are not human antibodies and they develop resistance to the use of antibodies as therapeutic agents. So scientist started to look for human cell lines to generate good amount specific kind of IgGs against a specific antigen.
Developing Humanized IgGs (HIgGs):
Several strategies were employed. First they isolated human B-lymphocytes by tagging fluorescent antigen and then they are separated by flow cytometry and got reasonably enriched population of specific B-lymphocyte. Then human antibody producing lymphocytes are fused with human myeloma cells. Unfortunately the hybrid cell lines were found to be unstable. Instead they transformed lymphocytes with Epstein Barr viruses. Such transformed cells were viable, they divided and secreted IgGs, but alas! The yield was very very poor.
The other strategy is to clone a human IgG gene, both L and H chains, and transform embryonic cells of the mouse and obtain the transgenic mouse and stimulate mouse for specific antigen and get specific antibodies.
In another method they obtained specific antibody gene for a specific antigen. By genetic manipulation it is possible to replace human Fc fragment in the place of mice Fc fragment. Similarly even the antigen binding hyper variable region can also be replaced. Such genes were transfected to known mammalian cell lines and expressed IgG were found to be human compatible. But it is yet to be realized to produce such Abs on large scale.
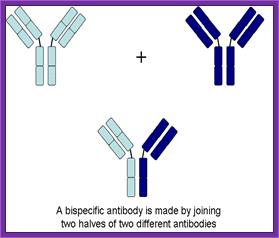
Use of Bispecific Antibodies:
Produce two different but specific antibodies; say one for tumor cell surface receptor and the other for specific T-cell receptor. When such antibodies were denatured and mixed to renature appropriately, one obtains antibodies containing two specificities called Bispecific antibodies.
Another way to generate Bispecific antibodies is to genetically manipulate hyper variable sites to the designed purpose. When such Bispecific antibodies are employed one can target two systems simultaneously. Bispecific antibodies, say one for specific T- killer cells and another for tumor cell, T-killer cells can be targeted to tumor cells.
In the case of malignant Hodgkin’s melanoma there are a large number of tumors infiltrating T-cells. And tumor cells have their own specific antigenic receptors. Raise antibodies separately for these two types of cells. Generate hybrid IgGs. Collect TIls (Tumor Infiltrating Lymphocytes) as much possible from the tumor and treat with specific Interleukin to activate the T-cells into killer cells. Inject T-killer cells and Bispecific antibodies. They bind to T-cell and bring them to tumor cells and bind. The activated Tills destroy tumors and clinically found such tumors were regressed.
Bispecific Monoclonal Antibodies: It is symbolized as BsMAB, BsAB,. It is lab made antibody. It can simultaneously bind to two different types of antigens. Current application is targeted to cancer immunotherapy and drug delivery. There are many format , but two of them are, one like IgG and another non-IgG like.
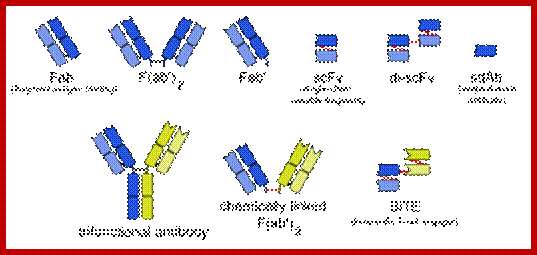
Three types of bispecific antibodies: trifunctional antibody, chemically linked Fab and bi-specific T-cell engager (bottom row). Blue and yellow parts distinguish parts from separate monoclonal antibodies. https://en.wikipedia.org
IgG like: It is like monoclonal antibody with two Fab arms and one Fc region. The most common and prevalent one is tri functional antibodies. These are produced with quadroma or hybrid hybridoma method.
The "knobs into holes" approach for manufacturing IgG-like bsMabs is shown on the left, while a diagram depicting the DVD-Ig format is on the right. The red dot indicates a possible site for introducing mutations in the heavy chain. Blue and yellow correspond to separate monoclonal antibodies. https://en.wikipedia.org
Non IgG like: There are other bsMabs that lack an Fc region completely. These are fusion proteins. there are various types of bivalent and trivalent single-chain variable fragments (scFvs). They have vartiable domains. The new formats are bi-specific T-cell engagers (BITEs).
How they act: they are exemplified by catumaxomab, representing the first approved bispecific- trifunctional antibody. Thery are very efficient used for cancer therapy.
The mechanism of action of a BsMAb, exemplified by catumaxomab, representing the first approved bispecific trifunctional antibody. https://en.wikipedia.org
Micromet - Biting Cancer (Part I):
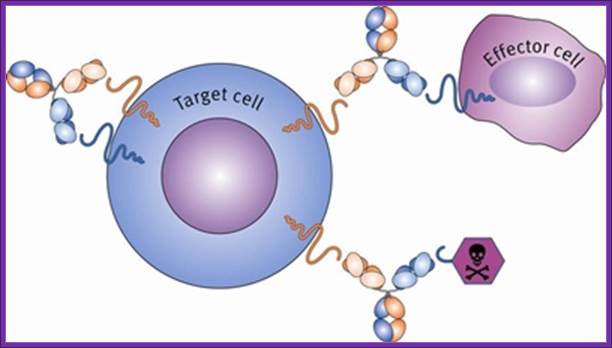
In the context of cancer therapy, Bispecific antibodies are usually designed to redirect immune cells to tumor cells, in a way that will lead to the attack of the tumor. One arm is directed against a target on a cancer cell while the other arm is directed against a target on the immune cell. In most cases, bispecific antibodies must not only bring the immune cell closer to cancer cells but also activate it to attack the tumor. In order to achieve both of these tasks, there is a need to identify specific targets on immune cells that can be activated by antibody binding, or find supplementary ways to achieve this activation. Naturally, there is a large number of options when designing a bsAb. One variable is the target on the cancer cells, another variable is the type of immune cell to be recruited, while a third variable is which structural element (antigen) on that specific immune cell should be targeted. This is much more complicated than designing mono-specific antibodies, in which only the target on the cancer cells should be chosen.
Genemab- Next Generation Technology:
Genmab is creating knowledge and intellectual property around Fab-arm exchange and the generation of bispecific antibodies, thereby establishing a strong basis for the DuoBody platform.
Developing fully human antibody therapeutics for the potential treatment of cancer; Gene Mabs; https://www.pharmatching.com
UniBodyTechnology:
UniBody is a proprietary antibody technology that creates a stable, smaller antibody format with an anticipated longer therapeutic window than current small antibody formats, based on pre-clinical studies to date. A UniBody molecule is about half the size of a regular type of inert antibody called IgG4 and binds with only one antibody arm to a therapeutic target. UniBody molecules are expected to be cleared from the body at a lower rate than other antibody fragments based on the preclinical studies to date. Unlike other antibodies which primarily work by killing targeted cells, a UniBody molecule will only inhibit or silence cells, which could be an advantage in the treatment of diseases such as asthma or allergies.
New antibody delivers a double blow ; 10:15 13 December 2003 by Andy Coghlan
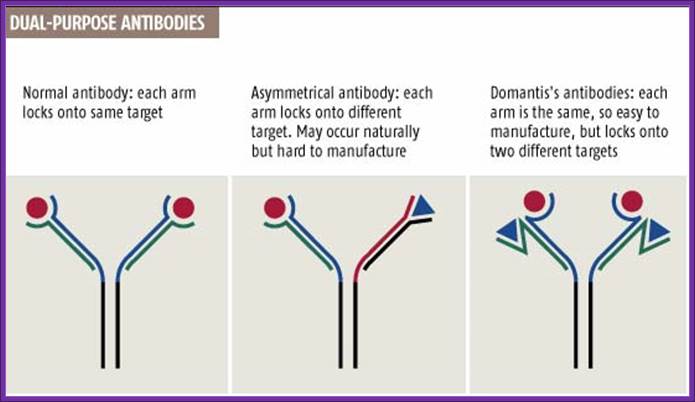
http://www.newscientist.com/
Normal antibodies have two identical arms, each made of two chains, that both bind to the same target (see graphic). But in 1999 Rob Aalberse, of the University of Amsterdam, reported that the body also produces dual-target antibodies, in which each arm locks onto a different target (New Scientist print edition, 18 September 1999).
Cancer cells, for instance, could be better targeted by antibodies that recognize two distinguishing features instead of just one. "With two targets, you'd enhance specificity, so sparing more healthy cells," says Tomlinson, who unveiled the method last week at an antibody conference in San Diego, California.
Epigenetically inducement of vigor in older people;
Transfusion of younger blood into older people has been subjected to studies, but it works in Mice. Where only blood without cells are pumped into older people particularly it is very helpful to older people with Alzheimer disease or for that matter anyone who is old wants to be young. Based on these trails certain people opened a company Alkahest to treat Alzheimer’s disease. Astonishingly this company is funded by the participants. Is this technology works or still in trails. If it works, how what the younger blood plasma contains’ these have to be worked out. Whether Dr.J. Karmazin’s Ambrosia’ trail works or not, there is a possibility and it should be tested. As transfusion of younger plasma does not cause any problem in the patient. As with experiments on mice has worked, why not in humans.
Application of Biotechnology and Gene Engineering in Medicine:
Gene engineering is the part of biotechnology, but specialization in gene isolation, sequencing, modifying and introducing into living system and disease diagnosis at biochemistry and molecular level.
Molecular Medicine 2017
Molecular medicine is a broad field, where physical, chemical, biological and medical techniques are used to describe molecular structures, mechanisms and identify fundamental molecular and genetic errors of disease, and to develop molecular interventions to correct them (Only few of them are discussed). It is a medical field that diagnoses the causes and provides treatment with medicines, surgery and often with gene therapy. Understanding of biochemistry of medicines and how the chemicals act at biochemical and molecular level is important (?) for Medical Doctors who treat patients. Typical applications of molecular medicines include gene therapy, molecular structural analysis, genetic epidemiology, and molecular and clinical pharmacology. This understanding of it requires good education in these areas in medical colleges by Doctors who have trained in such areas who have such knowledge; unfortunately this is deficient in most of our medical colleges who teach medicine (in India). Majority of doctors in our land have learnt disease diagnosis by symptoms and using stethoscope, prescribe medicines which are essentially remembered what medicine to what symptoms. Any practicing doctor who does not have pharmaceutical understanding of the drug is a sin!.
Typical applications in molecular medicine include gene therapy, molecular structural analysis, genetic epidemiology, and molecular and clinical pharmacology. In November 1949, with the seminal paper, "Sickle Cell Anemia, a Molecular Disease", in Science magazine, Linus Pauling, Harvey Itano and their collaborators laid the groundwork for establishing the field of molecular medicine. In 1956, Roger J. Williams wrote Biochemical Individuality, a prescient book about genetics, prevention and treatment of disease on a molecular basis, and nutrition which is now variously referred to as individualized medicine and orthomolecular medicine. Another paper in Science by Pauling in 1968, introduced and defined this view of molecular medicine that focuses on natural and nutritional substances used for treatment and prevention. Molecular medicine encompasses Biochemistry, Clinical Chemistry, Life Sciences, Medical Biology, Medical Chemistry, Medical Physics, Metabolomics, Molecular Biology, Molecular diagnostics and Molecular pathology are important components. www.wikipedia.org,
Molecular Medicine accomplishments in research have been recognized by the 2006 Nobel Prize in Physiology or Medicine awarded to Craig Mello (shared with Andrew Fire of Stanford University), the 2008 Lasker Basic Medical Research Award to Victor Ambros (shared with Gary Ruvkun of Harvard and David Baulcombe of Cambridge University), and the 2007 Medical Foundation Basic Science Award to David Lambright. Howard Hughes Medical Institute Investigators have done appointments to Michael Green, Roger Davis, and Craig Mello, and membership in the National Academy of Sciences (Mello and Ambros) and the Royal Society of London (Davis). Many other Molecular Medicine faculty have been recognized by awards for outstanding contributions in their fields of specialty, for example, a 2012 NIDA Avant Garde award to Jeremy Luban, the 2000 Banting Award to Michael Czech and the Elizabeth Glaser Scientist Award to Katherine Luzuriaga. Pew Scholar awards have been bestowed upon Tom Fazzio, Bert van den Berg and David Guertin. www.wikipedia.org, The Program in Molecular Medicine offers within its building a broad spectrum of state-of-the-art methodologies to its laboratory groups including deep sequencing, ultrafast 3D digital imaging microscopy (wide field and TIRF) of live cells, spinning disc confocal microscopy, x-ray crystallography, mouse metabolic phenotyping, mouse knockout technology and RNAi-based gene silencing in vitro and in vivo. Medical School Core facilities also make available a large number of additional technologies such as FACS analysis, gene profiling using microarrays, proteomics and both shRNA and small molecule screening. Expertise in chemistry, structural biology, biochemistry, cell and developmental biology, molecular biology, cell signaling and regulation, genomics and proteomics, bioinformatics, genetics, immunology and virology is strongly represented in the Program in Molecular Medicine. Program faculty members are also active in the teaching of these disciplines in both core and advanced courses for graduate and medical students. Structural biology at the UMass Medical School is supported by state-of-the-art X-ray and NMR core facilities housed in the Program in Molecular Medicine and the Department of Biochemistry and Molecular Pharmacology. Diffraction instrumentation includes three rotating anode X-ray generators equipped with R-axis IV, Mar 300 and Mar 350 image plates detectors, Osmic focusing mirrors, and nitrogen cryostreams. NMR instrumentation includes 400 MHz and 600 MHz Varian spectrometers equipped for multidimensional homonuclear and heteronuclear experiments. Computational resources include graphics workstations and multiprocessor Beowulf clusters for data processing, image reconstruction, 3D visualization, model building, refinement, molecular dynamics, and structural bioinformatics. Molecular Medicine laboratory groups utilize many model organisms in their research, including yeast, worms, flies, mice and nonhuman primates. Translational research on human subjects is also vigorously pursued with collaborators in clinical department www.wikipedia.org,
Mucosal immunology and vaccine; T Feng and C O Elson:
Mucosal immunology is one of the prominent techniques employed all over the world especially in under developed countries. Many of world scientist and great people like Bill Gates go to places where oral vaccine is required and demonstrate how people can use this technology, his contribution in terms of money to this field is remarkable and it has to be applauded. Starting from mouth, nose and ears to the end of large intestine is the largest mucosal surface. It is major surface that encounters all sorts of microorganisms and it has to combat when needed. This mucosal surface and is a major site of multifaceted interactions between the host mucosal immune system and components of the intestinal microbiota. Host immune responses to the commensal microbiota are tightly controlled and, meanwhile, the microbiota actively shapes intestinal immune responses to itself. Appreciation of these interactions during health and disease may direct therapeutic approaches to a broad range of autoimmune and inflammatory disorders in humans. In this review, we will discuss findings on how the intestinal immune system, especially adaptive immune cells, helps accommodate the large number of resident bacteria, and in turn how the microbiota shapes intestinal immune responses to achieve mutualism. http://www.nature.com
One of the key features of the intestinal immune system is its ability to distinguish between pathogenic and symbiotic bacteria, and thus protect against infection while avoiding detrimental and unnecessary inflammatory responses toward the normal microbiota. When these intestinal immune responses are dysregulated, they can result in chronic inflammatory disorders of the gut, including inflammatory bowel disease (IBD), celiac disease, and food allergies.
Host immune cells have developed a hierarchy of homeostatic mechanisms to ensure mucosal immune compartmentation and maintain systemic ignorance to commensal bacteria, including a layer of immunoglobulin (Ig) A- and antimicrobial peptide-containing mucus, a physical epithelial barrier, and innate and adaptive immune components. Understanding of the host–microbiota interactions during both steady-state homeostasis and pathological intestinal inflammation may help to direct therapeutic approaches to IBD as well as to a broad range of immune-mediated inflammatory disorders in humans.
Intestinal immune system, especially the adaptive immune component, resident microbiota, and in turn how the microbiota shapes intestinal immune responses to achieve mutualism. First, we understand the tight compartmentation of mucosal innate and adaptive immune responses, formed mainly by intestinal IgA reactive to microbial antigens, resulting in immune exclusion of the commensal microbial antigens and systemic immune “ignorance”. This will also highlight recent advances in understanding the influences of regulatory T (Treg) cells and effector T cells in the context of immune homeostasis and dysregulation, and feedback of the microbiota to intestinal T-cell regulation.
The human intestine harbors nearly 10x100 billion microorganisms composed of more than 1,000 distinct bacterial species as defined by high-throughput microbial 16S ribosomal RNA gene characterization.
First, the epithelial layer of the intestinal tract is formed by tightly connected intestinal epithelial cells and serves as a physical protective layer, separating luminal contents from the underlying immune compartments, and providing an efficient barrier to block the entry of microflora into the lamina propriety. Specialized intestinal epithelial cells such as mucus-secreting goblet cells and antimicrobial peptide-producing Paneth cells also contribute to the constitution of the mucosal barrier,
IgA, secreted by plasma cells and transported by intestinal epithelial cells into the lumen, is more abundant than the sum of all other Ig isotypes combined, and it joins the effort with bactericidal peptides in the mucus layer to form a passive defense line, which sequesters most resident bacteria in the lumen and dramatically reduces the microbial burden of the epithelium. The third layer of intestinal defense is formed by innate and adaptive immunity. Intestinal immune cells are extensively distributed throughout the intestinal mucosa, which is customarily divided into organized inductive and diffusely distributed effector sites.12 Innate and adaptive immune cells accumulate in these mucosal immune compartments and coordinate both to maintain a state of limited mucosal activation and to initiate active immune responses to invading microbes.
In contrast to the lack of concomitant systemic immune response, a strong intestinal IgA response to half of the rIBs and to two immunodominant microbiota flagellins, CBir1 and Flax, was identified, indicating a tight intestinal compartmentation of the active immune response to the microbiota, http://www.nature.com/2015-2106; , , and the mucosal immune system serves as the front-line defense against pathogens. It also tightly maintains immune tolerance to self-symbiotic bacteria, which are usually called commensals. Sensing both types of microorganisms is modulated by signaling primarily through various pattern-recognition receptors (PRRs) on barrier epithelial cells or immune cells. After sensing, proinflammatory molecules such as cytokines are released by these cells to mediate either defensive or tolerant responses. The interleukin-17 (IL-17) family members belong to a newly characterized cytokine subset that is critical for the maintenance of mucosal homeostasis. In this review, we will summarize recent progress on the diverse functions and signals of this family of cytokines at different mucosal edge.
The intestinal mucosa is a particularly dynamic environment in which the host constantly interacts with trillions of commensal microorganisms, known as the microbiota, and periodically interacts with pathogens of diverse nature. In this Review, we discuss how mucosal immunity is controlled in response to enteric bacterial pathogens, with a focus on the species that cause morbidity and mortality in humans. We explain how the microbiota can shape the immune response to pathogenic bacteria, and we detail innate and adaptive immune mechanisms that drive protective immunity against these pathogens. The vast diversity of the microbiota, pathogens and immune responses encountered in the intestines precludes discussion of all of the relevant players in this Review. Instead, we aim to provide a representative overview of how the intestinal immune system responds to pathogenic bacteria. http://www.nature.com/Araceli Perez-Lopez, Judith Behnsen et al; Nature Reviews Immunology.
· Mucosal immunity reduces the need for elimination of penetrating exogenous antigens by proinflammatory systemic immunity. The adult gut mucosa contains some 80% of the body's activated B cells-differentiated to plasma blasts and plasma cells (PCs). Most mucosal PCs produce dimeric immunoglobulin A (IgA), which, along with pentameric- immunoglobulin M (IgM), can be exported by secretory epithelia expressing the polymeric immunoglobulin receptor. Immune exclusion of antigens is performed mainly by secretory IgA in cooperation with innate defenses, but, in newborns and in IgA deficiency, secretory IgM is important. In the gut, induction and regulation of mucosal immunity occurs primarily in gut-associated lymphoid tissue-particularly the Peyer's patches-and also in mesenteric lymph nodes. Terminal differentiation to PCs is accomplished in the lamina propria to which the activated memory/effector T and B cells home. Lactating mammary glands are part of the secretory immune system, and IgA antibodies in breast milk reflect antigenic stimulation of gut-associated lymphoid tissue and nasopharynx-associated lymphoid tissue such as the tonsils. Breast-milk antibodies are thus highly targeted against infectious agents and other exogenous antigens in the mother's environment, which are those likely to be encountered by the infant. Therefore breast-feeding represents an ingenious immunologic integration of mother and child. https://www.ncbi.nlm.nih.gov.
Mucosal immunology is the study of immune system responses that occur at mucosal membranes of the intestines, the urogenital tract and the respiratory system, i.e., surfaces that are in contact with the external environment. In healthy states, the mucosal immune system provides protection against pathogens but maintains a tolerance towards non-harmful commensual microbes and benign environmental substances. It provides three main functions: protecting the mucous membrane against infection; preventing the uptake of antigens, microorganisms, and other foreign materials; and moderating the organism's immune response.
At birth, the neonate's mucosal immune system is relatively undeveloped, but the colonization of intestinal flora promotes its development. Because of its front-line status within the immune system, the mucosal immune system is being investigated for use in vaccines for various afflictions, including AIDS and allergies, collectively intestinal mucosa covers 400M666^2 area 400m2 (equivalent to one and a half tennis court) http://www.scq.ubc.ca/
The mucous membranes produce a special type of antibody called secretory IgA or sIgA. In the mucous, this antibody is secreted as a dimer, joined at the non-antigen binding end by a protein known as the J chain as shown in Figure.

Figure . Structure of secretory IgA. It consists of at least two IgA molecules covalently linked by a J chain and the secretory component, which is added as the antibody crossed the mucosal epithelial cells into the lumen. http://www.scq.ubc.ca/.
This form of the antibody is more stable, less resistant to proteolysis by the digestive enzymes of the gut, and has higher avidity for mucosal surfaces. The mucous membranes are bathed in huge quantities of sIgA, which act as a first line of defense to neutralize invading pathogens. Experimental evidence shows that the presence of sIgA correlates with resistance to infection by various pathogens, including bacteria, viruses, parasites and fungi. It has also been shown to neutralize viruses and prevent their adherence to the epithelial cells lining the mucous (thereby preventing infection) as well as mediating excretion of pathogens and preventing the assembly of mature virus particles.
Another important component of mucosal immunity is the T cell-mediated immune response. T cells that specifically recognize pathogens can help antibodies to clear the infection or directly kill the invader themselves. T cells produced in the mucous are capable of traveling throughout the mucosal tissues through special “homing” receptors on their membranes. This means that if an immune response is generated in the gastrointestinal lining, T cells produced there can travel to other mucosal sites, for example, the lungs or nasal cavity, providing protection over a large surface area;
Among the newer vaccines designed to induce a protective immune response to HIV is one designed to mimic the mucosal responses seen in these resistant individuals. In 2001, a US group tested the first mucosal HIV vaccine in rhesus macaques. While the vaccine showed promising results, but it failed to prevent infection.
· Organs of the immune system include the thymus, spleen, and lymph nodes. T-lymphocytes develop in the thymus, which is located in the chest directly above the heart. The spleen, which is located in the upper abdomen, makes antibodies and removes old and damaged red blood cells. The immune system is broadly divided into two major components: innate immunity and adaptive immunity. Innate immunity involves immediate, nonspecific responses to foreign invaders, while adaptive immunity requires more time to develop its complex, specific responses http://lpi.oregonstate.edu/.
Vaccination is one of the most successful applications of immunology and for a long time has depended on parenteral administration protocols. However, recent studies have pointed to the promise of mucosal vaccination because of its ease, economy and efficiency in inducing an immune response not only systemically, but also in the mucosal compartment where many pathogenic infections are initiated. However, successful mucosal vaccination requires the help of an adjuvant for the efficient delivery of vaccine material into the mucosa and the breaking of the tolerogenic environment, especially in oral mucosal immunization. Given that M cells are the main gateway to take up luminal antigens and initiate antigen-specific immune responses, understanding the role and characteristics of M cells is crucial for the development of successful mucosal vaccines. Especially, particular interest has been focused on the regulation of the tolerogenic mucosal microenvironment and the introduction of the luminal antigen into the lymphoid organ by exploiting the molecules of M cells. Here, we review the characteristics of M cells and the immune regulatory factors in mucosa that can be exploited for mucosal vaccine delivery and mucosal immune regulation. Sae-Hae Kim and Yong-Suk Jang; http://www.nature.com/

Expression of mucosal IgA immune responses after different routes of vaccination: http://www.nature.com/
The 'common mucosal immune system' is more restricted than previously thought. In humans, immunization studies with cholera toxin B subunit by different mucosal routes have clearly shown that the strongest response takes place at the directly vaccine-exposed mucosa and the second-best responses at adjacent mucosae or at specifically interconnected inductive-expression mucosal systems such as the gut-mammary gland link in lactating women. A notable exception is the fact that nasal mucosal immunization not only stimulates an immune response in the respiratory tract, but also can give rise to a strong genital-vaginal mucosal immune response. Shading indicates strength of response.
Regulatory Mechanisms: The mucosal immune system has evolved a variety of mechanisms to achieve and maintain tolerance against self-antigens and against the plethora of environmental antigens present in the microflora, in food and among airborne matter. Studies in animal models have identified that mucosal tolerance can be achieved through different mechanisms, including activation-induced cell death, anergy and, most important, the induction of regulatory T cells. Anergy of antigen-specific T cells has been reported after inhalation or ingestion of large quantities of soluble proteins, and deletion of specific T cells only after mucosal administration of massive, non-physiological antigen doses42. Induction of regulatory cells after mucosal delivery of antigens has been reported in animal models for more than 25 years and has received a major attention during the last few years given the potential of such regulatory cells as therapeutic agents in immune-mediated diseases.
In mice, four main types of regulatory T cells
have been described: (i) antigen-induced CD4+TH2-like
cells that produce IL-4 and IL-10 and antagonize the activity of TH1
effector cells; (ii) CD4+CD45RBlow Tr1 cells that function through the
production of IL-10; (iii) CD4+ or
CD8+ T cells producing
TGF-![]() (TH3
cells); and apparently most important, (iv) a population of naturally
occurring CD4+CD25+ regulatory
T cells (Treg cells)
that suppress proliferation through a cell contact−dependent mechanism.
Although anergic in vitro,
the latter cells can be expanded in an antigen-specific manner in vivo after immunization. Notably, these
cells may also confer suppressor activity on other CD4+ T cells by inducing the expression of
the transcription factor Foxp3 and/or the major histocompatibility complex
(MHC) class II−binding molecule LAG-3 in such cells ('infectious
tolerance')51, 52. Thereby, they may also provide a direct
link between effector T-cell inhibition by Treg, TH3 and
Tr1 cells. Thus, natural human CD4+CD25+ Treg expressing the mucosal
(TH3
cells); and apparently most important, (iv) a population of naturally
occurring CD4+CD25+ regulatory
T cells (Treg cells)
that suppress proliferation through a cell contact−dependent mechanism.
Although anergic in vitro,
the latter cells can be expanded in an antigen-specific manner in vivo after immunization. Notably, these
cells may also confer suppressor activity on other CD4+ T cells by inducing the expression of
the transcription factor Foxp3 and/or the major histocompatibility complex
(MHC) class II−binding molecule LAG-3 in such cells ('infectious
tolerance')51, 52. Thereby, they may also provide a direct
link between effector T-cell inhibition by Treg, TH3 and
Tr1 cells. Thus, natural human CD4+CD25+ Treg expressing the mucosal ![]() 4
4![]() 7 integrin, when
co-cultured with conventional CD4+ T
cells, induced Tr1-like IL-10−secreting T cells with strong suppressor
activity on effector T cells, whereas another,
7 integrin, when
co-cultured with conventional CD4+ T
cells, induced Tr1-like IL-10−secreting T cells with strong suppressor
activity on effector T cells, whereas another, ![]() 4
4![]() 1-positive Treg subset in similar cultures instead
induced TH3-like TGF-
1-positive Treg subset in similar cultures instead
induced TH3-like TGF-![]() −secreting suppressor
T cells51. Recent evidence indicates that all of
these different regulatory T cell types and mechanisms can be induced or
expanded by mucosal administration of antigens leading to peripheral tolerance
(oral tolerance; J.-B. Sun et al., unpublished data).
−secreting suppressor
T cells51. Recent evidence indicates that all of
these different regulatory T cell types and mechanisms can be induced or
expanded by mucosal administration of antigens leading to peripheral tolerance
(oral tolerance; J.-B. Sun et al., unpublished data).
Organs of the immune system include the thymus, spleen, and lymph nodes. T-lymphocytes develop in the thymus, which is located in the chest directly above the heart. The spleen, which is located in the upper abdomen, makes antibodies and removes old and damaged red blood cells. The immune system is broadly divided into two major components: innate immunity and adaptive immunity. Innate immunity involves immediate, nonspecific responses to foreign invaders, while adaptive immunity requires more time to develop its complex, specific responses, http://lpi.oregonstate.edu/.
Oral Vaccines; Polio vaccine; Trivalent oral poliovirus vaccine (tOPV); The trivalent vaccine was withdrawn in April 2016 and replaced with the bivalent oral poliovirus vaccine (bOPV), which contains only attenuated virus of types 1 and 3. This is because continued use of tOPV threatened to continue seeding new type 2 circulating vaccine-derived polioviruses (cVDPV2), despite the wild type 2 virus being eradicated in 1999.
The first polio vaccine was the inactivated polio vaccine; It was developed by Jonas Salk and came into use in 1955. The oral polio vaccine was developed by Albert Sabin and came into commercial use in 1961; The wholesale cost in the developing world is about 0.25 USD per dose for the oral form as of 2014. In the United States it costs between 25 and 50 USD for the inactivated form. OPV also proved to be superior in administration, eliminating the need for sterile syringes and making the vaccine more suitable for mass vaccination campaigns. OPV also provided longer lasting immunity than the Salk vaccine.

http://polioeradication.org/- live attenuated vaccine-virus in OPV
The oral polio vaccine is simple to administer. A few drops, given multiple times, can protect a child for life. © WHO/Rod Curtis. OPV also proved to be superior in administration, eliminating the need for sterile syringes and making the vaccine more suitable for mass vaccination campaigns. OPV also provided longer lasting immunity than the Salk vaccine.
One dose of OPV produces immunity to all three poliovirus serotypes in approximately 50% of recipients. Three doses of live-attenuated OPV produce protective antibodies to all three poliovirus types in more than 95% of recipients. OPV produces excellent immunity in the intestine, the primary site of wild poliovirus entry, which helps prevent infection with wild virus in areas where the virus is endemic. The live virus used in the vaccine is shed in the stool and can be spread to others within a community. The live virus also has stringent requirements for transport and storage, which are a problem in some hot or remote areas. As with other live-virus vaccines, immunity initiated by OPV is probably lifelong. The trivalent (against wild type 1, 2 and 3) OPV has been used and nearly eradicated polio infection worldwide. Spearheaded by The Global Polio Eradication Initiative, 155 countries switched to use the bivalent (against wild type 1 and 3) between 17 April and 1 May 2016.
The Salk vaccine, or inactivated poliovirus vaccine (IPV), is based on three wild, virulent reference strains, Mahoney (type 1 poliovirus), MEF-1 (type 2 poliovirus), and Saukett (type 3 poliovirus), grown in a type of monkey kidney tissue culture (Vero cell line), which are then inactivated with formalin. The injected Salk vaccine confers IgG-mediated immunity in the bloodstream, which prevents polio infection from progressing to viremia and protects the motor neurons, thus eliminating the risk of bulbar polio and post-polio syndrome.
In United States, the vaccine was administered along with the diphtheria, tetanus, and acellular pertussis vaccines (DTaP) and a pediatric dose of hepatitis B vaccine. In the UK, IPV is combined with tetanus, diphtheria, pertussis, and Haemophilus influenzae type b vaccines
Sabin strains were chosen for worldwide distribution. There are 57 nucleotide substitutions which distinguish the attenuated Sabin 1 strain from its virulent parent (the Mahoney serotype), two nucleotide substitutions attenuate the Sabin 2 strain, and 10 substitutions are involved in attenuating the Sabin 3 strain. The primary attenuating factor common to all three Sabin vaccines is a mutation located in the virus's internal ribosome entry site (IRES); In 1961, type 1 and 2 monovalent oral poliovirus vaccine (MOPV) was licensed, and in 1962, type 3 MOPV was licensed. In 1963, trivalent OPV (TOPV) was licensed, and became the vaccine of choice in the United States and most other countries of the world, largely replacing the inactivated polio vaccine. A second wave of mass immunizations led to a further dramatic decline in the number of polio cases. Between 1962 and 1965 about 100 million Americans (roughly 56% of the population at that time) received the Sabin vaccine. The result was a substantial reduction in the number of poliomyelitis cases, even from the much reduced levels following the introduction of the Salk vaccine.
Indian-The trial also demonstrated the superiority of bOPV compared to the respective Sabin type 1 and 3 strains contained in the trivalent oral poliovirus vaccine (tOPV). Immediately after the trial results were available, the ACPE convened by conference call and reviewed the trial outcomes and current epidemiology of wild poliovirus globally.
Mucosa:
An ulcer (/ˈʌlsər/; from Latin ulcus, "ulcer, sore") is a break in the skin or mucous membrane with loss of surface tissue and the disintegration and necrosis of epithelial tissue.
Mucosal immunity and vaccines:
There is currently great interest in developing mucosal vaccines against a variety of microbial pathogens. Mucosally induced tolerance also seems to be a promising form of immunomodulation for treating certain autoimmune diseases and allergies. Here we review the properties of the mucosal immune system and discuss advances in the development of mucosal vaccines for protection against infections and for treatment of various inflammatory disorders.www.nature.com
The mucosal immune system has three main functions: (i) to protect the mucous membranes against colonization and invasion by potentially dangerous microbes that may be encountered, (ii) to prevent uptake of not degraded antigens including foreign proteins derived from ingested food, airborne matter and commensal microorganisms, and (iii) to prevent the development of potentially harmful immune responses to these antigens if they do reach the body interior. At variance with the systemic immune apparatus, this functions in a normally sterile milieu and often responds vigorously to invaders, the MALT guard’s organs that are replete with foreign matter. It follows that upon encountering this plethora of antigenic stimuli; the MALT must economically select appropriate effector mechanisms and regulate their intensity to avoid bystander tissue damage and immunological exhaustion.
GENERIC NAME(S): Guaifenesin is an expectorant. It works by thinning and loosening mucus in the airways, clearing congestion, and making breathing easier.
Mucosal immunity and vaccines:
The main mucosa-associated lymphoid tissues (MALT) of teleosts are the gut-associated lymphoid tissue (GALT), skin-associated lymphoid tissue (SALT), the gill-associated lymphoid tissue (GIALT) and the recently discovered nasopharynx-associated lymphoid tissue (NALT). Teleost MALT includes diffuse B cells and T cells with specific phenotypes different from their systemic counterparts that have co-evolved to defend the microbe-rich mucosal environment. Both B and T cells respond to mucosal infection or vaccination. Irene Salinas; Both B and T cells respond to mucosal infection or vaccination. Specific antibody responses can be measured in the gills, gut and skin mucosal secretions of teleost fish following mucosal infection or vaccination. Rainbow trout studies have shown that IgT antibodies and IgT+ B cells are the predominant B cell subset in all MALT and respond in a compartmentalized manner to mucosal infection. Our current knowledge on adaptive immunity in teleosts is limited compared to the mammalian literature. New research tools and in vivo models are currently being developed in order to help reveal the great intricacy of teleost mucosal adaptive immunity and help improve mucosal vaccination protocols for use in aquaculture. http://www.mdpi.com/
Polio vaccine:
The oral polio vaccine is simple to administer. A few drops, given multiple times, can protect a child for life. © WHO/Rod Curtis; http://polioeradication.org/- live attenuated vaccine-virus in OPV . For example, many vaccines contain sodium and potassium salt based which are essential for life. People may think of formaldehyde as a man-made chemical, but in small quantities it is also found naturally in the bloodstream. These are the Active ingredients of the vaccine made from viruses or bacteria (also called ‘antigens’). A few vaccines in the UK schedule are made using recombinant DNA technology; genetically modified organisms (GMOs) is used . Few vaccines contain Aluminium (an adjuvant), Thiomersal, also called Thimerosal (a preservative), Gelatine (a stabiliser), emulsifiers, sugar, Lactose, Mannitol , latex, glycerol, glutamate, Egg Proteins (Ovalbumin), acidity regulators are added-Dec 20, 2016. Oral adjuvents-polio-Type1, type 2 and type 3-40, 8 and 32 units of antigens-expients antibiotics (neomycin, streptomycin and polymyxin are also used. Some vaccines contain 2-phenoxyethanol as a preservative. Thiomersal cannot be used for IPV. http://www.who.int/vaccine_safety
Trivalent oral poliovirus vaccine (tOPV);
The trivalent vaccine was withdrawn in April 2016 and replaced with the bivalent oral poliovirus vaccine (bOPV), which contains only attenuated virus of types 1 and 3. This is because continued use of tOPV threatened to continue seeding new type 2 circulating vaccine-derived polioviruses (cVDPV2), despite the wild type 2 virus being eradicated in 1999.
The first polio vaccine was the inactivated; It was developed by Jonas Salk and came into use in 1955. The oral polio vaccine was developed by Albert Sabin and came into commercial use in 1961; The wholesale cost in the developing world is about 0.25 USD per dose for the oral form as of 2014. In the United States it costs between 25 and 50 USD for the inactivated form. OPV also proved to be superior in administration, eliminating the need for sterile syringes and making the vaccine more suitable for mass vaccination campaigns. OPV also provided longer lasting immunity than the Salk vaccine. Attenuated IPV and bPV inactivated Polio and bPVBivalent PV are in use.
The Salk vaccine, or inactivated poliovirus vaccine (IPV), is based on three wild, virulent reference strains, Mahoney (type 1 poliovirus), MEF-1 (type 2 poliovirus), and Saukett (type 3 poliovirus), grown in a type of monkey kidney tissue culture (Vero cell line), which are then inactivated with formalin. The injected Salk vaccine confers IgG-mediated immunity in the bloodstream, which prevents polio infection from progressing to viremia and protects the motor neurons, thus eliminating the risk of bulbar polio and post-polio syndrome.
In the United States, vaccine is administered along with the diphtheria, tetanus, and acellular pertussis vaccines (DTaP) and a pediatric dose of hepatitis B vaccine. In the UK, IPV is combined with tetanus, diphtheria, pertussis, and Haemophilus influenzae type b vaccines
Sabin strains were chosen for worldwide distribution. There are 57 nucleotide substitutions which distinguish the attenuated Sabin 1 strain from its virulent parent (the Mahoney serotype), two nucleotide substitutions attenuate the Sabin 2 strain, and 10 substitutions are involved in attenuating the Sabin 3 strain. The primary attenuating factor common to all three Sabin vaccines is a mutation located in the virus's internal ribosome entry site (IRES); In 1961, type 1 and 2 monovalent oral poliovirus vaccine (MOPV) was licensed, and in 1962, type 3 MOPV was licensed. In 1963, trivalent OPV (TOPV) was licensed, and became the vaccine of choice in the United States and most other countries of the world, largely replacing the inactivated polio vaccine. A second wave of mass immunizations led to a further dramatic decline in the number of polio cases. Between 1962 and 1965 about 100 million Americans (roughly 56% of the population at that time) received the Sabin vaccine. The result was a substantial reduction in the number of poliomyelitis cases, even from the much reduced levels following the introduction of the Salk vaccine.
Indian made OPV trial also demonstrated the superiority of bOPV compared to the respective Sabin type 1 and 3 strains contained in the trivalent oral poliovirus vaccine (tOPV). Immediately after the trial results were available, the ACPE convened by conference call and reviewed the trial outcomes and current epidemiology of wild poliovirus globally.
Plant based polio mucosoal vaccine https://www.sciencedaily.com/
Edible vaccine production for veterinary use has received widespread attention because of health initiatives aimed at decreasing antibiotic use in livestock and other animals to avoid the development of antibiotic-resistant strains, especially of epidemic and zoonotic pathogens. These issues have promoted the development of plant-based vaccines, which can easily fulfil these requirements (zoonotic).
Edible vaccines are actually recombinant vaccines in which selected antigens against a particular pathogen are introduced into a plant and grown. Oral delivery of this plant induces a protective immune response against that particular pathogen in the form of an edible vaccine; Plant cells expressing vaccine antigens or biopharmaceuticals can be lyophilized and stored at ambient temperature for many years maintaining efficacy of expressed protein drugs; Plant-based vaccines have the potential to induce a mucosal immune response and a systemic immune response without the pain and risk associated with needles and injections.
Banana vaccine: Inspired by calf fed on banana- made vaccine in Banana; not succeded, Redig Mandy. Plant vaccines will be a billion-dollar business, said Professor Arntzen, who leads a team of the world's top plant scientists at Cornell University in New York State. Successful experiments show that the vaccine worked, he added, and will cost less than one penny a dose to make. A single gene transferred into a tomato or banana plant is reproduced as a protein thousands of times inside the fruit. When eaten it passes into the intestine and then into the blood stream producing antibodies against hepatitis B - working the same way as a traditionally injected but much more expensive vaccine. A single dried banana chip or tomato paste sandwiched in a wafer contains enough protein to act as a vaccine dose. Traditional vaccines have to be refrigerated and cost £10 for a successful course, making them too expensive for developing countries where hepatitis B kills many people directly and causes the death of many more through subsequent cancer. Tomatoes and bananas genetically modified to contain hepatitis B vaccine could rid the world of the virus, a leading American scientist said in London yesterday. Based on this plant based oral vaccine one can develop many such vaccines against a large number of infecting microbes. https://www.theguardian.com;
Edible vaccine production for veterinary use has received widespread attention because of health initiatives aimed at decreasing antibiotic use in livestock and other animals to avoid the development of antibiotic-resistant strains, especially of epidemic and zoonotic pathogens. These issues have promoted the development of plant-based vaccines, which can easily fulfil these requirements (zoonotic).
Edible vaccines are actually recombinant vaccines in which selected antigens against a particular pathogen are introduced into a plant. Oral delivery of this plant induces a protective immune response against that particular pathogen in the form of an edible vaccine; Plant cells expressing vaccine antigens or biopharmaceuticals can be lyophilized and stored at ambient temperature for many years maintaining efficacy of expressed protein drugs; Plant-based vaccines have the potential to induce a mucosal immune response and a systemic immune response without the pain and risk associated with needles and injections Banana vaccine: Redig Mandy ; Institution: Biochemistry- inspired by calf fed on banana-made vaccine in Banana; Redig Mandy; not come into the market.
Plant vaccines will be a billion-dollar business, said Professor Arntzen, who leads a team of the world's top plant scientists at Cornell University in New York state. Successful experiments show that the vaccine worked, he added, and will cost less than one penny a dose to make. A single gene transferred into a tomato or banana plant is reproduced as a protein thousands of times inside the fruit. When eaten it passes into the intestine and then into the blood stream producing antibodies against hepatitis B - working the same way as a traditionally injected but much more expensive vaccine. A single dried banana chip or tomato paste sandwiched in a wafer contains enough protein to act as a vaccine dose. Traditional vaccines have to be refrigerated and cost £10 for a successful course, making them too expensive for developing countries where hepatitis B kills many people directly and causes the death of many more through subsequent cancer. Tomatoes and bananas genetically modified to contain hepatitis B vaccine could rid the world of the virus, a leading American scientist said in London. https://www.theguardian.com; Transgenic potato that expresses the hepatitis B surface antigen which can be used as vaccine.. http://www.news.cornell.edu/ Transgenic potato that expresses the hepatitis B surface antigen which can be used as vaccine. http://www.news.cornell.edu/
Bayer; GMO foods, Genetically modified foods; sugar, aspartame Papaya, canola, cotton Dairy corn, soybean, sugar beets, potatoes, Tomatoes, squash, oils, golden rice, Zucchini, apple, papaya, squash, corn, sugar beets, Jatropha, BT cotton, wine. Rape seed, vitamins, vegetables, tobacco, peas they are the few of them.
Banana Vaccine-Yasmin Thanavala; BANANA plants and TOMATO plants growing at the Boyce Thompson Institute for Plant Research at Cornell University have been genetically engineered to produce vaccines in their fruit. Bananas are particularly appealing as vaccines because they grow widely in many parts of the developing world, can be eaten raw and are liked by most children. If such plants are made available to common men, they can grow such plant in their yard and whenever required by anyone can be used. https://www.mcdb.ucla.edu
The advantages would be enormous. The plants could be grown locally, and cheaply, using the standard growing methods of a given region. Because many food plants can be regenerated readily, the crops could potentially be produced indefinitely without the growers having to purchase more seeds or plants year after year. Homegrown vaccines would also avoid the logistical and economic problems posed by having to transport traditional preparations over long distances, keeping them cold en route and at their destination. And, being edible, the vaccines would require no syringes— which, aside from costing something, can lead to infections if they become contaminated
JARED SCHNEIDMAN DESIGN; Cut leaf. Expose leaf to bacteria carrying an antigen gene and an antibiotic- resistance gene. Allow bacteria to deliver the genes into leaf cells. Expose leaf to an antibiotic to kill cells that lack the new genes. Wait for surviving (gene-altered) cells to multiply and form a clump (callus). Then allow callus to sprout shoots and roots. 5 Put in soil. Within three months, the plantlets will grow into plants bearing antigen-laden vaccine potatoes. https://www.mcdb.ucla.edu
Tumor necrosis factor (TNF); Treatment for certain tumor cells-Tumor necrosis factor (TNF, tumor necrosis factor alpha, (cachexin, or cachectin) is a cell signaling protein (cytokine) involved in systemic inflammation and is one of the cytokines that make up the acute phase reaction; it is produced chiefly by activated macrophages, although it can be produced by many other cell types such as CD4+ lymphocytes, NK cells, neutrophils, mast cells, eosinophils, and neurons. Dysregulation of TNF production has been implicated in a variety of human diseases including Alzheimer's disease, cancer, major depression, psoriasis and inflammatory bowel disease (IBD).
TNF Cell Signaling:
TNF can bind two receptors, TNFR1 (TNF receptor type 1; CD120a; p55/60) and TNFR2 (TNF receptor type 2; CD120b; p75/80). TNFR1 is 55-kDa and TNFR2 is 75-kDa. TNFR1 is expressed in most tissues, and can be fully activated by both the membrane-bound and soluble trimeric forms of TNF, whereas TNFR2 is found typically in cells of the immune system, and respond to the membrane-bound form of the TNF homo-trimer. As most information regarding TNF signaling is derived from TNFR1, the role of TNFR2 is likely underestimated.

https://en.wikipedia.orgSignalling Pathway:
TNF can bind two receptors, TNFR1 (TNF receptor type 1; CD120a; p55/60) and TNFR2 (TNF receptor type 2; CD120b; p75/80). TNFR1 is 55-kDa and TNFR2 is 75-kDa. TNFR1 is expressed in most tissues, and can be fully activated by both the membrane-bound and soluble trimeric forms of TNF, whereas TNFR2 is found typically in cells of the immune system, and respond to the membrane-bound form of the TNF homotrimer. As most information regarding TNF signaling is derived from TNFR1, the role of TNFR2 is likely underestimated. https://en.wikipedia.org;
TNF – TNF is a Protein Kinase: 1. Pathways signaling through PI 3-kinase and phosphatidylinositol (3,4,5)P3 (PI-3 kinase and protein kinase B/Akt). 2. Mitogen-activated protein kinases (MAPKinases). NB: Both group 1and 2 signals also activate protein kinase Cγ and Protein kinase Cζ. 3. Possible interaction via kinases not coupled to IRS proteins.
It has been suggested that the most dominant is the first group (PI 3-kinase) which converts phosphatidylinositol 3,4 bisphosphate (PIP2) or [PI(3,4)P2] to phosphatidylinositol 3,4,5 triphosphate PIP3 or (PI 3,4,5)P3. These nucleotides act as anchors, binding down-line protein kinases to the plasma membrane and activating them. These nucleotides seem to be responsible for the alterations in carbohydrate, protein and lipid metabolism that are initiated by insulin.
It is now clear that a serine-threonine kinase "Akt" (otherwise known as protein kinase B) is THE central element in the actions of insulin. Akt is activated by PIP3. Recent work has shown that PIP3 binding opens Akt to phosphorylation bu phosphoinositide-dependent protein kinase PDK-1 and the mammalian target of rapamycin (mTOR) complex 2 (mTORC2). Look at the next figure from the work of Professor Peter Shepherd (Acta Physiol Scand 183, 3-12, 2005). Phosphorylation of the insulin receptor and IRS1 and 2 lead to binding and activation of phosphatidylinositol 3 kinase (PI3K) and formation of PI(3,4,5)P3. This then binds to the plasma membrane and associates with phosphoinositol- dependent kinase-1 (PDK-1) and leading to phosphorylation and activation of PKB/Akt. Activated Akt is initiates many of the metabolic actions of insulin.
This gene encodes a multifunctional proinflammatory cytokine that belongs to the tumor necrosis factor (TNF) superfamily. This cytokine is mainly secreted by macrophages. It can bind to, and thus functions through its receptors TNFRSF1A/TNFR1 and TNFRSF1B/TNFBR. This cytokine is involved in the regulation of a wide spectrum of biological processes including cell proliferation, differentiation, apoptosis, lipid metabolism, and coagulation. This cytokine has been implicated in a variety of diseases, including autoimmune diseases, insulin resistance, and cancer. Knockout studies in mice also suggested the neuroprotective function of this cytokine. [RefSeq, Jul 2008] https://www.ncbi.nlm.nih.gov
TNF gene maps to chromosome 6p21.3, spans about 3 kilobases and contains 4 exons. The last exon codes for more than 80% of the secreted protein.[21] The 3' UTR of TNFα contains an AU-rich element (ARE). TNF protein is primarily produced as a 233-amino acid-long type II transmembrane protein arranged in stable homotrimers; heterotrimeric. From this membrane-integrated form the soluble homotrimeric cytokine (sTNF) is released via proteolytic cleavage by the metalloprotease TNF alpha converting enzyme (TACE, also called ADAM17). The soluble 51 kDa trimeric sTNF tends to dissociate at concentrations below the nanomolar range, thereby losing its bioactivity. The secreted form of human TNFα takes on a triangular pyramid shape, and weighs around 17-kD. Both the secreted and the membrane bound forms are biologically active, although the specific functions of each is controversial. But, both forms do have overlapping and distinct biology activities
This gene encodes a multifunctional proinflammatory cytokine that belongs to the tumor necrosis factor (TNF) superfamily. This cytokine is mainly secreted by macrophages. It can bind to, and thus functions through its receptors TNFRSF1A/TNFR1 and TNFRSF1B/TNFBR. This cytokine is involved in the regulation of a wide spectrum of biological processes including cell proliferation, differentiation, apoptosis, lipid metabolism, and coagulation. This cytokine has been implicated in a variety of diseases, including autoimmune diseases, insulin resistance, and cancer. Knockout studies in mice also suggested the neuroprotective function of this cytokine. [provided by RefSeq, Jul 2008] https://www.ncbi.nlm.nih.gov
Interleukin-2 (IL-2). Cancer treatment, immune deficiency, and HIV infection treatment; Interleukin-2 is the only drug approved in the US for the treatment of metastatic RCC. It is also approved in many other countries. But IL-2 isn't just a drug. IL-2 is a natural part of your immune system, a messenger protein called a cytokine which activates parts of your immune system. IL-2 does not kill tumor cells directly like classical chemotherapy. Instead, IL-2 activates and stimulates the growth of immune cells, most importantly T-Cells, but also Natural Killer Cells (NK Cells), both of which are capable of destroying cancer cells directly.
The term interleukin derives from (inter-) "as a means of communication", and (-leukin) "deriving from the fact that many of these proteins are produced by leukocytes and act on leukocytes". "as a means of communication". They are used in Cancer treatment, immune deficiency, and HIV infection; Interleukin-2 is the only drug approved in the US for the treatment of metastatic RCC. It is also approved in many other countries. But IL-2 isn't just a drug. IL-2 is a natural part of your immune system, a messenger protein called a cytokine which activates parts of your immune system. IL-2 does not kill tumor cells directly like classical chemotherapy. Instead, IL-2 activates and stimulates the growth of immune cells, most importantly T-Cells, but also Natural Killer Cells (NK Cells), both of which are capable of destroying cancer cells directly. Some interleukins are classified as lymphokines, lymphocyte-produced cytokines that mediate immune responses.
Human T- and B-cells recognize invaders by the shape of molecules - antigens - on their surfaces. Our immune system can produce a T- and B-cell to fit every possible shape. However, any T- or B-cell that recognized molecules found on your cells were destroyed while you were growing in the womb, to prevent them from attacking your own body. But you were left with millions of others, one for every foreign antigen you might ever encounter.
Having recognized the invader, different types of T-cell then have different jobs to do. Some send chemical instructions (cytokines) to the rest of the immune system. Your body can then produce the most effective weapons against the invaders, which may be bacteria, viruses or parasites. Other types of T-cells recognize and kill virus-infected cells directly. Some help B-cells to make antibodies, which circulate and bind to antigens.
With the help of T-cells, B-cells make special Y-shaped proteins called antibodies. Antibodies stick to antigens on the surface of germs, stopping them in their tracks, creating clumps that alert your body to the presence of intruders. Your body then starts to make toxic substances to fight them. Patrolling defender cells called phagocytes engulf and destroy antibody-covered intruders.
T cells (thymus cells) and B cells (bone marrow- or bursa-derived cells [a]) are the major cellular components of the adaptive immune response. T cells are involved in cell-mediated immunity, whereas B cells are primarily responsible for humoral immunity (relating to antibodies). http://www.sciencemuseum.org.uk/



IFB gamma and Interferon Alfa and Interferon Beta and Recombinant IFN alpha 2B- respectively; https://www.dreamstime.com

Typical Interferon-IL6; https://en.wikipedia.org/

Immuno-Cytokine; Cytokine-a fusion protein- http://www.roche.com
Immunoglobulin? http://www.sciencemuseum.org.uk

Antibody-cytokine fusion proteins, which consist of cytokines fused to an antibody, have the properties of all components and acquire advantages compared to proteins alone. Structurally, there are two main types of ACFPs which cytokines can be alternatively fused to either whole immunoglobulin (Ig fusion) or antigen-binding fragments such as Fabs, single-chain variable fragments (scFvs) or divalent derivatives thereof, e.g. diabodies. Moreover, differences in the composition of the fusion proteins are also due to the cytokine itself. Many cytokines are composed of a single monomeric protein domain (e.g. IL-2 and IFN-a), while others are homodimeric (e.g. IL-10 and IFN-γ), homotrimeric molecules (e.g. TNF and TRAIL) or heterodimeric (e.g. IL-12 and IL-27). Each cytokine can be fused at the amino- or carboxy-terminus of the antibody, which depends on the structure of the cytokine and antibody, and in order to conserve the biological activity of both components. Further improvements also include the fusion of different cytokines to an antibody. Cytokines include chemokines, interferons, interleukins, lymphokines, and tumour necrosis factors; Antibody-cytokine fusion proteins; Creative biolabs-http://www.creativebiolabs.net/
What interferon and interleukin are?
Immune function is helped by two kinds of white blood cells. The “B cells” (so-called because they develop in bone marrow) produce antibodies. The “T cells” (so-called because they develop in a small organ called the thymus gland) are responsible for a variety of other immune responses. Interferon and interleukin are substances made by cells in the body, to communicate with each other. Interferons (IFNs) are low molecular weight proteins that belong to the class of glycoproteins known as cytokines. They act as signaling molecules released by host cells in response to pathogens such as viruses, bacteria, parasites and even tumour cells. So tumour cells are foreigners for the body. IFNs are part of the non-specific immune system and are an important first line of defense against viral infections. They are released by host cells in response to the presence of pathogens such as viruses, bacteria, parasites or tumor cells. IFNs have other functions: they activate immune cells, such as natural killer cells and macrophages; they increase recognition of infection or tumor cells by up-regulating antigen presentation to T lymphocytes; and they increase the ability of uninfected host cells to resist new infection by virus. FNs belong to the large class of proteins known as cytokines, molecules used for communication between cells to trigger the protective defenses of the immune system that help eradicate pathogens. Interferons are named for their ability to "interfere" with viral replication by protecting cells from virus infections. IFNs also have various other functions: they activate immune cells, such as natural killer cells and macrophages; they increase host defenses by up-regulating antigen presentation by virtue of increasing the expression of major histocompatibility complex (MHC) antigens. Certain symptoms of infections, such as fever, muscle pain and "flu-like symptoms", are also caused by the production of IFNs and other cytokines. Host symptoms, such as aching muscles and fever, are related to the production of IFNs during infection. They are used to treat diseases such as Multiple Sclerosis (MS) and Hepatitis Interferon and interleukin can boost the immune system, so doctors sometimes use man made versions to treat cancer. Because of the way it works, doctors sometimes call this type of treatment immunotherapy. Some viruses are resistant to Interferons.
Production of interferons occurs mainly in response to microbes, such as viruses and bacteria, and their products. Binding of molecules uniquely found in microbes—viral glycoproteins, viral RNA, bacterial endotoxin (lipopolysaccharide), bacterial flagella, CpG motifs—by pattern recognition receptors, such as membrane bound Toll like receptors or the cytoplasmic receptors RIG-I or MDA5, can trigger release of IFNs. Toll Like Receptor 3 (TLR3) is important for inducing interferons in response to the presence of double-stranded RNA viruses; the ligand for this receptor is double-stranded RNA (dsRNA). After binding dsRNA, this receptor activates the transcription factors IRF3 and NF-κB, which are important for initiating synthesis of many inflammatory proteins. RNA interference technology tools such as siRNA or vector-based reagents can either silence or stimulate interferon pathways. Release of IFN from cells (specifically IFN-γ in lymphoid cells) is also induced by mitogens. Other cytokines, such as interleukin 1, interleukin 2, interleukin-12, tumor necrosis factor and colony-stimulating factor, can also enhance interferon production. Interferon therapy is used (in combination with chemotherapy and radiation) as a treatment for some cancers. This treatment can be used in hematological malignancy; leukemia and lymphomas including hairy cell leukemia, chronic myeloid leukemia, nodular lymphoma, and cutaneous T-cell lymphoma. Patients with recurrent melanomas receive recombinant IFN-α2b. Both hepatitis B and hepatitis C are treated with IFN-α, often in combination with other antiviral drugs. Some of those treated with interferon have a sustained virological response and can eliminate hepatitis virus. The most harmful strain—hepatitis C genotype I virus—can be treated with a 60-80% success rate with the current standard-of-care treatment of interferon-α, ribavirin and recently approved protease inhibitors such as Telaprevir (Incivek) May 2011, Boceprevir (Victrelis) May 2011 or the nucleotide analog polymerase inhibitor Sofosbuvir (Sovaldi) December 2013. Biopsies of patients given the treatment show reductions in liver damage and cirrhosis. Some evidence shows giving interferon immediately following infection can prevent chronic hepatitis C, although diagnosis early in infection is difficult since physical symptoms are sparse in early hepatitis C infection. Control of chronic hepatitis C by IFN is associated with reduced hepatocellular carcinoma.
The term 'interleukin' (IL) has been used to describe a group of cytokines with complex immunomodulatory functions - including cell proliferation, maturation, migration and adhesion. These cytokines also play an important role in immune cell differentiation and activation. Determining the exact function of a particular cytokine is complicated by the influence of the producing cell type, the responding cell type and the phase of the immune response. ILs can also have pro- and anti-inflammatory effects, further complicating their characterization. These molecules are under constant pressure to evolve due to continual competition between the host's immune system and infecting organisms; as such, ILs have undergone significant evolution. This has resulted in little amino acid conservation between orthologous proteins, which further complicates the gene family organization.
Interleukins are a large group of immunomodulatory proteins that elicit a wide variety of responses in cells and tissues. These cytokines comprise a large number of the known immunological 'second messenger' molecules within mammals. Interleukins initiate a response by binding to high-affinity receptors located on the surface of cells; Interleukins function in a paracrine or autocrine fashion, rather than as an endocrine signal, which is more common with steroidal and amino acid-derived hormones. The response of a particular cell to these cytokines depends on the ligands involved, specific receptors expressed on the cell surface and the particular signalling cascades that are activated. ILs modulate growth, differentiation and activation during an immune response. This distinguishes them from chemokines - the main function of which is to direct immune cells to the site of inflammation via chemotaxis - and interferons (IFNs), which predominantly mediate cellular response to viral infection. Despite attempts to separate these three groups based on function, there is a degree of overlap.
How ILs work; Interferon and interleukin work in several ways, including; one of several proteins important for lymphocyte proliferation.Interleukin-Interfering with the way cancer cells grow and multiply
· Stimulating the immune system and encouraging killer T cells and other cells to attack cancer cells
· Encouraging cancer cells to produce chemicals that attract immune system cells to them
Doctors use interferon alpha for several different types of cancer, particularly
· Kidney cancer (renal cell cancer)
· Melanoma
· Some types of leukaemia
· You can have interferon alpha into the bloodstream through a drip (infusion). But you are more likely to have it as an injection just under the skin (subcutaneously). How often you have it depends on which type of cancer you are having treatment for. Most people have interferon 3 times a week but you can have it as a daily injection.
Some of the side effects that interferon alpha and interleukin 2 can cause include; Fatigue (tiredness and weakness), Flu like symptoms, Diarrhoea, Low levels of blood cells, Feeling sick and Loss of appetite; Interleukin 2 can also cause low blood pressure. http://www.cancerresearchuk.org.
There are several types of T-Cells but, without going into detail, certain T-Cells are capable of killing tumor cells if they recognize a specific antigen on the surface of the tumor cell. Antigens are normally proteins. Each T-Cell is specific for only one antigen but you have many different T-Cells. NK Cells have the ability to kill tumor cells without needing to recognize a specific antigen (I'm not sure how!). While this sounds good, NK cells are weaker cancer killers than T-Cells. The so called LAK cells which were used in some of the early immunotherapy experiments are actually NK cells. There are human 36-37 interleukins in human beeings, produced by different cell types. http://www.cancerguide.org/
There are several types of T-Cells but, without going into detail, certain T-Cells are capable of killing tumor cells if they recognize a specific antigen on the surface of the tumor cell. Antigens are normally proteins. Each T-Cell is specific for only one antigen but you have many different T-Cells. NK Cells have the ability to kill tumor cells without needing to recognize a specific antigen (I'm not sure how!). While this sounds good, NK cells are weaker cancer killers than T-Cells. The so called LAK cells which were used in some of the early immunotherapy experiments are actually NK cells. http://www.cancerguide.org/
The term 'interleukin' (IL) has been used to describe a group of cytokines with complex immunomodulatory functions - including cell proliferation, maturation, migration and adhesion. These cytokines also play an important role in immune cell differentiation and activation. Determining the exact function of a particular cytokine is complicated by the influence of the producing cell type, the responding cell type and the phase of the immune response. ILs can also have pro- and anti-inflammatory effects, further complicating their characterization. These molecules are under constant pressure to evolve due to continual competition between the host's immune system and infecting organisms; as such, ILs have undergone significant evolution. This has resulted in little amino acid conservation between orthologous proteins, which further complicates the gene family organization.
Interleukins are a large group of immunomodulatory proteins that elicit a wide variety of responses in cells and tissues. These cytokines comprise a large number of the known immunological 'second messenger' molecules within mammals. Interleukins initiate a response by binding to high-affinity receptors located on the surface of cells; Interleukins function in a paracrine or autocrine fashion, rather than as an endocrine signal, which is more common with steroidal and amino acid-derived hormones. The response of a particular cell to these cytokines depends on the ligands involved, specific receptors expressed on the cell surface and the particular signalling cascades that are activated. ILs modulate growth, differentiation and activation during an immune response. This distinguishes them from chemokines - the main function of which is to direct immune cells to the site of inflammation via chemotaxis - and interferons (IFNs), which predominantly mediate cellular response to viral infection. Despite attempts to separate these three groups based on function, there is a degree of overlap.
The term 'interleukin' (IL) has been used to describe a group of cytokines with complex immunomodulatory functions - including cell proliferation, maturation, migration and adhesion. These cytokines also play an important role in immune cell differentiation and activation. Determining the exact function of a particular cytokine is complicated by the influence of the producing cell type, the responding cell type and the phase of the immune response. ILs can also have pro- and anti-inflammatory effects, further complicating their characterisation. These molecules are under constant pressure to evolve due to continual competition between the host's immune system and infecting organisms; as such, ILs have undergone significant evolution. This has resulted in little amino acid conservation between orthologous proteins, which further complicates the gene family organisation.
Interleukins are a large group of immunomodulatory proteins that elicit a wide variety of responses in cells and tissues. These cytokines comprise a large number of the known immunological 'second messenger' molecules within mammals. Interleukins initiate a response by binding to high-affinity receptors located on the surface of cells; Interleukins function in a paracrine or autocrine fashion, rather than as an endocrine signal, which is more common with steroidal and amino acid-derived hormones. The response of a particular cell to these cytokines depends on the ligands involved, specific receptors expressed on the cell surface and the particular signalling cascades that are activated. ILs modulate growth, differentiation and activation during an immune response. This distinguishes them from chemokines - the main function of which is to direct immune cells to the site of inflammation via chemotaxis - and interferons (IFNs), which predominantly mediate cellular response to viral infection. Despite attempts to separate these three groups based on function, there is a degree of overlap.
Interferons; (Treatment for cancer and viral infections); Interferons (IFNs) are a group of signaling proteins made and released by host cells in response to the presence of several pathogens, such as viruses, bacteria, parasites, and also tumor cells. In a typical scenario, a virus-infected cell will release interferons causing nearby cells to heighten their anti-viral defenses. IFNs belong to the large class of proteins known as cytokines, molecules used for communication between cells to trigger the protective defenses of the immune system that help eradicate pathogens. Interferons are named for their ability to "interfere" with viral replication; by protecting cells from virus infections. IFNs also have various other functions: they activate immune cells, such as natural killer cells and macrophages; they increase host defenses by up-regulating antigen presentation by virtue of increasing the expression of major histocompatibility complex (MHC) antigens. Certain symptoms of infections, such as fever, muscle pain and "flu-like symptoms", are also caused by the production of IFNs and other cytokines. More than twenty distinct IFN genes and proteins have been identified in animals, including humans. They are typically divided among three classes: Type I IFN, Type II IFN, and Type III IFN. IFNs belonging to all three classes are important for fighting viral infections and for the regulation of the immune system. https://en.wikipedia.org/wiki
The mammalian types are designated IFN-α (alpha), IFN-β (beta), IFN-κ (kappa), IFN-δ (delta), IFN-ε (epsilon), IFN-τ (tau), IFN-ω (omega), and IFN-ζ (zeta, also known as limitin).
IFNs of different kinds also have various other functions: they activate immune cells, such as natural killer cells and macrophages; they increase host defenses by up-regulating antigen presentation by virtue of increasing the expression of major histocompatibility complex (MHC) antigens. Certain symptoms of infections, such as fever, muscle pain and "flu-like symptoms", are also caused by the production of IFNs and other cytokines.
More than twenty distinct IFN genes and proteins have been identified in animals, including humans. They are typically divided among three classes: Type I IFN, Type II IFN, and Type III IFN. IFNs belonging to all three classes are important for fighting viral infections and for the regulation of the immune system
Type 1 interferons (IFN-1) are implicated in the pathogenesis of systemic lupus erythematosus (SLE), but most studies have only reported the effect of IFN-1 on mixed cell populations. We aimed to define modules of IFN-1-associated genes in purified leucocyte populations and use these as a basis for a detailed comparative analysis.
Production of type I interferon (IFN) is an essential component of the innate immune response against invading pathogens. However, its production must be tightly regulated to avoid harmful effects. Compounds that modulate the IFN response are potentially valuable for a variety of applications due to IFN’s beneficial and detrimental roles. We developed and executed a cell-based high-throughput screen (HTS) targeting components that participate in and/or regulate the IRF3 and nuclear factor (NF)–κB branches of the IFN induction pathway. The assay detects activation of the IFN induction pathway via an enhanced green fluorescent protein (eGFP) reporter gene under the control of the IFNβ promoter and was optimized, miniaturized, and demonstrated suitable for HTS as robust Z′ factor scores of >0.6 were consistently achieved. A diversity screening set of 15,667 small molecules was assayed and two novel hit compounds validated that specifically inhibit the IFN induction pathway. We demonstrate that one of these compounds acts at or upstream of IRF3 phosphorylation. A second cell-based assay to detect activation of the IFN signaling (Jak-Stat) pathway via an eGFP reporter gene under the control of an IFN-stimulated response element (ISRE) containing MxA promoter also performed well (robust Z′ factor >0.7) and may therefore be similarly used to identify small molecules that modulate the IFN signaling pathway;
Interferon beta-1a and interferon beta-1b: are used to treat and control multiple sclerosis, an autoimmune disorder. Interferon therapy is used (in combination with chemotherapy and radiation) as a treatment for some cancers. It is recently demonstrated that type I interferon (IFN-I) signaling was responsible for many of the immune dysfunctions associated with persistent virus infection and in particular the induced expression of the suppressive factors IL-10 and PDL1 by dendritic cells (DCs). Yet, mechanistically how IFN-I signaling specifically generates and programs cells to become immunosuppressive is still unknown. Herein, we define the underlying mechanisms of IFN-I mediated immunosuppression and establish that the induction of factors and the generation of the DCs that express them are separable events integrally reliant on additional inflammatory factors. Further, we demonstrate a similar derivation of the suppressive DCs that emerge in other diseases associated with prolonged inflammation and immunosuppression, specifically in HIV infection, Mycobacterium tuberculosis, and cancer, indicating a conserved origin of immunosuppression and suggesting that targeting the pathways that underlie expression of immunosuppressive cells and factors could be beneficial to treat multiple chronic diseases. https://www.ncbi.nlm.nih.gov/ PLoS Pathog. Zoe O. Gage 2016 Jan; Cameron R. Cunningham,1 Ameya Champhekar et al.
|
Pharmaceutical forms of interferons |
|
|
Generic name |
Trade name |
|
Interferon alpha 2a |
|
|
Intron A/Reliferon/Uniferon |
|
|
Human leukocyte Interferon-alpha (HuIFN-alpha-Le) |
|
|
Interferon beta 1a, liquid form |
|
|
Interferon beta 1a, lyophilized |
|
|
Interferon beta 1a, biogeneric (Iran) |
|
|
Betaseron / Betaferon |
|
|
PEGylated interferon alpha 2a (Egypt) |
Reiferon Retard |
|
PEGylated interferon alpha 2b plus ribavirin (Canada) |
Pegetron |
IFNA1, IFNA2, IFNA4, IFNA5, IFNA6, IFNA7.’, IFNA8, IFNA10, IFNA13, IFNA14, IFNA16, IFNA17’ IFNA21, IFNB1, IFNW, IFNE1, IFNK; https://en.wikipedia.org/wiki/Interferon; The above mentioned are some of the Interferons.
Interferon beta (IFNβ) is a cytokine that is naturally produced by the immune system in response to biological and chemical stimuli. It signals by binding to the heterodimeric type I IFN receptor composed of the IFNAR1 and IFNAR2 chains, and regulates the expression of a plethora of genes by means of the classical JAK/STAT and other pathways. IFNβ is pleiotropic in that it elicits antiviral, antiproliferative, and immunomodulatory activities on numerous cell types. The biological activities underpin the mechanisms by which the protein is used to treat various diseases such as hepatitis C infection and multiple sclerosis. Despite the success of IFNβ therapy, the drug may evoke the production of antidrug antibodies that may reduce treatment efficiency. Immunogenicity is related to many factors: among them, structural properties, particularly aggregation. and T-cell and B-cell epitopes in the structure of IFNβ, appear to be important. Knowledge of the structural properties of IFNβ and its relation to immunogenicity may help scientists to develop safer and more effective forms. Several methods have been used to predict and reduce the immunogenicity of certain IFNβ drug products. In this chapter, we review the current knowledge on IFNβ from its structure, dynamic conformation, signaling pathway, and mechanism of action to its therapeutic effects. Immunogenicity and its relation to structural properties of IFNβ are also discussed. https://www.researchgate,
Interferon (Treatment for cancer and viral infections); Interferons (IFNs) are a group of signaling proteins made and released by host cells in response to the presence of several pathogens, such as viruses, bacteria, parasites, and also tumor cells. https://en.wikipedia.org/wiki In a typical scenario, a virus-infected cell will release interferons causing nearby cells to heighten their anti-viral defenses. IFNs belong to the large class of proteins known as cytokines, molecules used for communication between cells to trigger the protective defenses of the immune system that help eradicate pathogens. Interferons are named for their ability to "interfere" with viral replication, by protecting cells from virus infections. IFNs also have various other functions: they activate immune cells, such as natural killer cells and macrophages; they increase host defenses by up-regulating antigen presentation by virtue of increasing the expression of major histocompatibility complex (MHC) antigens. Certain symptoms of infections, such as fever, muscle pain and "flu-like symptoms", are also caused by the production of IFNs and other cytokines. More than twenty distinct IFN genes and proteins have been identified in animals, including humans. They are typically divided among three classes: Type I IFN, Type II IFN, and Type III IFN. IFNs belonging to all three classes are important for fighting viral infections and for the regulation of the immune system. https://en.wikipedia.org/wiki/Interferon
IFN-γ is mostly secreted by activated CD4 + , CD8 + T cells and NK cells. This cytokine has immunomodulatory, anti-cancer and anti-microbial effects and is important for prophylaxis, diagnosis and treatment of chronic infections and cancers. Objective: The purpose of this study was to clone the full cDNA of human IFN-γ and express it in CHO cell line. Methods: Lymphocytes from a healthy individual were isolated and activated by phytohaemagglutinin (PHA) in vitro. After 4 hours, total RNA extracted and first cDNA strand was synthesized. cDNA was amplified with primers containing EcoRI and NotI sites. The amplified fragment and the PcDNA3.1 vector were cut by EcoRI and NotI and ligated. The construct (pcDNA3.1-IFN-γ) was transferred into E.coli (DH5α strain) using CaCl2 method and selected by plating on a medium containing ampicillin. The construct sequence was confirmed by PCR and sequence analysis. Construct expression was achieved by performing a calcium phosphate-mediated transfection into CHO cells and followed by selection of stable drug (G418) resistant clones by limiting dilution assay (LDA). The IFN-γ production by transfected CHO cells was measured using ELISA technique. Results and Conclusion: Out of 33 grown transformed bacterial colonies, only 6 had the entire sequences of the inserted fragment and one of them was used for the transfection experiment. Out of 768 wells, 5 clones produced more than 100 ng/ml/10 6 cells of IFN-γ. Among the 5 clones, one with the maximum production of INF-γ (143 ng/ml/10 6 cells) was selected and used for propagation.
IFN-α[edit]
The IFN-α proteins are produced by leukocytes. They are mainly involved in innate immune response against viral infection. The genes responsible for their synthesis come in 13 subtypes that are called IFNA1, IFNA2, IFNA4, IFNA5, IFNA6, IFNA7, IFNA8, IFNA10, IFNA13, IFNA14, IFNA16, IFNA17, IFNA21. These genes are found together in a cluster on chromosome 9.
IFN-α is also made synthetically as medication in hairy cell leukemia. The International Nonproprietary Name (INN) for the product is interferon alfa. The recombinant type is interferon alfacon-1. The pegylated types are pegylated interferon alfa-2a and pegylated interferon alfa-2b.
IFN-β
The IFN-β proteins are produced in large quantities by fibroblasts. They have antiviral activity that is involved mainly in innate immune response. Two types of IFN-β have been described, IFN-β1 (IFNB1) and IFN-β3 (IFNB3) (a gene designated IFN-β2 is actually IL-6). IFN-β1 is used as a treatment for multiple sclerosis as it reduces the relapse rate.
IFN-β1 is not an appropriate treatment for patients with progressive, non-relapsing forms of multiple sclerosis.[6]
IFN-ε, -κ, -τ, -δ, and -ζ]
IFN-ε, -κ, -τ, and -ζ appear, at this time, to come in a single isoform in humans, IFNK. Only ruminants encode IFN-τ, a variant of IFN-ω. So far, IFN-ζ is only found in mice, while a structural homolog, IFN-δ is found in a diverse array of non-primate and non-rodent placental mammals. Most but not all placental mammals encode functional IFN-ε and IFN-κ genes.
IFN-ω
IFN-ω, although having only one functional form described to date (IFNW1), has several pseudogenes: IFNWP2, IFNWP4, IFNWP5, IFNWP9, IFNWP15, IFNWP18, and IFNWP19 in humans. Many non-primate placental mammals express multiple IFN-ω subtypes
IFN-ν- [not to be confused with Interferon gamma (IFNγ)]
This subtype of Type I IFN was recently described as a pseudogene in human, but potentially functional in the domestic cat genome. In all other genomes of non-feline placental mammals, IFN-ν is a pseudogene; in some species, the pseudogene is well preserved, while in others, it is badly mutilated or is undetectable. Moreover, in the cat genome, the IFN-ν promoter is deleteriously mutated. It is likely that the IFN-ν gene family was rendered useless prior to mammalian diversification. Its presence on the edge of the Type I IFN locus in mammals may have shielded it from obliteration, allowing its detection.
Sources and functions:
IFN-α and IFN-β are secreted by many cell types including lymphocytes (NK cells, B-cells and T-cells), macrophages, fibroblasts, endothelial cells, osteoblasts and others. They stimulate both macrophages and NK cells to elicit an anti-viral response, and are also active against tumors. Plasmacytoid dendritic cells have been identified as being the most potent producers of type I IFNs in response to antigen, and have thus been coined natural IFN producing cells. Current study findings suggest that by forcing IFN-α expression in tumor-infiltrating macrophages, it is possible to elicit a more effective dendritic cell activation and immune effector cell cytotoxicity.[7]

https://en.wikipedia.org/
IFN-α acts as a pyrogenic factor by altering the activity of thermosensitive neurons in the hypothalamus thus causing fever. It does this by binding to opioid receptors and eliciting the release of prostaglandin-E2 (PGE2).
A similar mechanism is used by IFN-α to reduce pain; IFN-α interacts with the μ-opioid receptor to act as an analgesic.; IFN-ω is released by leukocytes at the site of viral infection or tumors.
In mice, IFN-β inhibits immune cells to produce growth factors, thereby slowing tumor growth, and inhibits other cells from producing vessel producing growth factors, thereby blocking tumor angiogenesis and hindering the tumour from connecting into the blood vessel system;
Human Interferon;
. Author links open the author workspace.. Author links open the author workspace. http://www.sciencedirect.com/We describe a universal ligand-binding receptor for human interferons α and interferon β (type I IFNs). A soluble 40 kDa IFN-αβ receptor (p40) that blocks the activity of type I IFNs was purified from urine and sequenced. Antibodies raised against p40 completely block the activity of several type I IFNs and immunoprecipltate both a cellular 102 kDa IFN-αβ receptor and its cross-linked complexes with IFN-α2. The receptor is a disulfide-linked dimer, consisting of 51 kDa subunits. We isolated and expressed a 1.5 kb cDNA, coding for the IFN-αβ receptor. Its 331 amino acid sequence includes a leader and a transmembrane region, while its ectodomain corresponds to p40. IFN-αβ receptor is physically associated with the cytoplasmic Tyr kinase JAK1, hence, in addition to ligand binding, it is directly involved in signal transduction.
Interferon α2b gene was amplified by PCR using specific primers containing appropriate restriction enzymes, plant highly expression sequence and Histidine tag sequence. Target sequence was cloned in plant expression vector pCAMBIA1304 and the construct named pCAMINFα. pCAMINFα was transferred to E. coli strain DH5α and plated on LB agar medium containing kanamycin 50 mgl-1. The colonies were confirmed by colony PCR and sequencing. The construct was transferred into Agrobacterium tumefaciens by freeze-thaw method and transformed colonies were confirmed by colony PCR. Tobacco plants (cultivar xanthi) were inoculated with A. tumefaciens strain LBA4404 by leaf disc method. Inoculated explants were cultured on MSII (MS + BAP 1mgl-1 + NAA 0.1 mgl-1) at 28°C and darkness for 48 hours. Then explants were transferred to selection medium containing cephotaxime (250 mgl-1) and hygromycin (15 mgl-1) in a 16/8 (day/night) h photoperiod in growth room with an irradiance of 5000 lux. Transgenic plants were regenerated and transferred to perlite. Genomic DNA was extracted from regenerated plants by Dellaporta method at 5-leaf step and transgenic lines were confirmed by PCR with specific primers. Expression of Interferon α2b gene was confirmed by dot blotting. https://www.ncbi.nlm.nih.gov Shahrzad Ahangarzadeh et al.
A cDNA encoding the human interferon-γ receptor was isolated from a λgt11 expression library using a polyclonal antireceptor antiserum. The gene for this receptor was identified in a cosmid library and transfected into mouse cells. The human interferon-γ receptor expressed in mouse cells displayed the same binding properties as in human cells. However, transfected cells were not sensitive to human IFN-γ, suggesting the need for species-specific cofactors in receptor function. As inferred from the cDNA sequence, the human interferon-γ receptor shows no similarities to known proteins and represents a novel transmembrane receptor. It is most likely the product of a single mRNA and a gene located on chromosome 6q. http://www.sciencedirect.com
Diabetes: Sushruta (6th century BCE) identified diabetes and classified it as Madhumeha. He further identified it with obesity and sedentary lifestyle, advising exercises to help "cure" it. The ancient Indians tested for diabetes by observing whether ants were attracted to a person's urine, and called the ailment "sweet urine disease" (Madhumeha).
Genetically Engineered Pharmaceuticals- Insulins:
Insulin for diabetics- by itself N HORMONE; required for the action of other major mammalian hormones that promote growth. Three groups of molecules possibly related to this anabolic function are considered: (1) molecules that relate to the structure of membranes and the initiation of insulin action, including insulin receptors; (2) molecules (such as cyclic nucleotides and cations, e.g. K+ and Mg+ + ) that are potentially concerned with transmission and amplification of information from the plasma membrane to subcellular functional units; (3) molecules that may contribute directly to development of juvenile or maturity-onset diabetes. Two new concepts are proposed: First, the receptor for insulin occupies a locus on the plasma membrane coupled to Mg++-activated (Na+ + K+ )- ATPase; hormone activation stimulates ATPase activity. As a result of the activation of the membrane ATPase enzyme system by insulin, concentrations of K+ and Mg + + increase at critical intracellular loci; these ions then serve as "second messengers." The second new" concept is that the genetic abnormality in human diabetes mellitus lies in a single or multiple enzyme defect that leads to accumulation of a fragment from growth hormone. One such fragment is known to inhibit glucose uptake and fatty acid synthesis.
Insulin and glucagon are two hormones regulating glucose and fat metabolism in the body. Both are synthesized in the pancreas. Both are proteins, but physiologically they are opposites.
Insulin is a protein hormone. It contains 51 amino acids. It weighs 5808 Daltons (a unit of weight measurement). It is made up of two protein chains linked together by a disulfide bond. A gene called INS codes for the precursor of insulin is preproinsulin. Pancreatic cells called beta cells secrete insulin. These cells are located in clusters called islets of Langerhan.
High blood sugar level promotes the release of insulin from beta cells while stress hormones (adrenalin) inhibit insulin release. In healthy individuals, pancreas secretes insulin in tightly controlled amounts to maintain the blood glucose levels within normal parameters. Insulin is critical in regulating carbohydrates and lipids. It regulates glucose, amino acid, and lipid absorption by cells all over the body. It increases DNA replication and protein synthesis. The action of insulin is widespread but more pronounced in liver, muscle cells, and fat tissue. Liver and skeletal muscle tissues store glucose as glycogen while fat tissue stores it as triglycerides under the influence of insulin. Insulin promotes glycogen synthesis, lipid synthesis, and fat esterification; therefore, glycogen breakdown and fat breakdown occur when insulin levels are low. The body hydrolyses glycogen (a stored form of glucose) to release glucose into the blood stream when blood sugar drops below normal levels. Insulin inhibits the secretion of glucagon which has the opposite action of insulin. It also inhibits the use of lipid as an energy source. The blood level of insulin acts as a signal to alter the direction of biochemical reactions in cells. It also inhibits sodium excretion by kidneys.
Glucogon: Glucagon is a protein hormone. It contains 29 amino acids. It weighs 3485 Daltons. Genes code for the precursor of glucagon is proglucagon; which is then cleaved into the active form of glucagon in alpha cells of pancreas. But in the intestines proglucagon breaks down to form different products. Low blood sugar level, stress hormones like adrenalin, amino acids like Arginine, Alanine, neurotransmitters like acetylcholine, and hormones like cholecystokinin increase glucagon secretion. Human growth inhibiting hormones, insulin, and urea inhibit glucagon secretion. Glucagon increases blood sugar level. It promotes glycogenolysis. Even though glucagon promotes glucose synthesis from fatty acids it does not affect fat breakdown.
Therapeutic uses of glucagon include relaxation of the lower esophageal sphincter in the esophageal blocks and spasms, severe hypoglycemia, and for treating beta blocker overdose.
What is the difference between Insulin and Glucagon?
Both are protein hormones. High blood sugar level promotes insulin secretion while inhibiting glucagon secretion. • Stress hormones inhibit insulin secretion while promoting glucagon secretion. • The beta cells secrete insulin while the alpha cells secrete glucagon. • Insulin reduces blood sugar while glucagon increases. • Insulin forces substances (glucose, amino acids) into cells while glucagon inhibits it. • Insulin promotes the synthesis of glycogen while glucagon breaks glycogen down. • Insulin promotes lipid synthesis, but glucagon does not break it down. • Insulin inhibits glucagon formation while glucagon does not control insulin secretion.
Main points: When blood glucose levels increase over about 5 mmol/l the beta-cells increase their output of insulin and C-peptide. The glucagon-producing alpha-cells remain quiet, and hold on to their hormone. A fall in blood glucose under about 4 mmol/l leads to a pronounced decrease in insulin secretion. The alpha-cells become active and deliver glucagon to the blood.

http://www.medbio.info
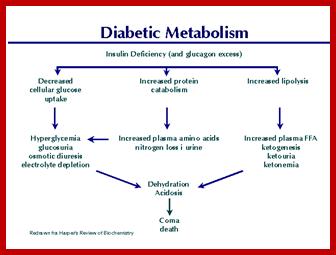
http://medboio.info
Hypothalamic KATP Channels Regulate Hepatic Gluconeogenesis and Postpranial Blood Glucose Levels:
Blood sugar levels are dependent upon glucose uptake after meals and hepatic release of glucose between meals. The sugar released from the liver comes either from stored glycogen or production of glucose from lactate and amino acids. This production of glucose is largely responsible for stabilization of postprandial blood sugar levels. The hyperglycemia noted in type 2 diabetes partially results from lack of control over hepatic glucose formation due to resistance to insulin. It has recently become clear that part of this insulin effect occurs indirectly through insulin-sensitive receptors in the brain (more precisely, in the hypothalamus). In a very recent article in Nature, Alessandro Pocai and coauthors presented convincing data that couples insulin-stimulation of hypothalamic KATP channels with neural control of hepatic gluconeogenesis (Nature 434, 1026-1031, 2005; and an overview by Nature's editors ((click here)). Insulin stimulated opening of hypothalamic KATP channels results in vagal nerve signaling to the liver and inhibition of gluconeogenesis. This is part of the normal response to meals and following insulin release from the pancreatic ß-cells. Thus, signaling from the brain is one of the important control mechanisms which establish correct "between-meal" blood sugar levels. Hypothalamic insulin resistance and therefore loss of control over hepatic gluconeogenesis may well be one of the important factors involved in development of type 2 diabetes. This model is summarized in this figure from the overview in Nature . (Nature 434, 1026-31, 2005
It stimulates 1. Transport of glucose ions, amino acids, 2. Glycogen formation, 3. Glucose conversion to triglycerides .4. Nucleic acid synthesis and protein synthesis.

http://www.alluvionbiomedical.com/

Fig Diet-induced physiological changes in the liver that promote improved blood glucose control. http://www.alluvionbiomedical.com/
Insulin for diabetics- It is estimated that 450 million people suffer from this disease. By itself is an HORMONE; required for the action of other major mammalian hormones that promote growth. It is now genetically engineered protein available in the market all over the world. Three groups of molecules possibly related to this anabolic function are considered: (1) molecules that relate to the structure of membranes and the initiation of insulin action, including insulin receptors; (2) molecules (such as cyclic nucleotides and cations, e.g. K+ and Mg+ + ) that are potentially concerned with transmission and amplification of information from the plasma membrane to subcellular functional units; (3) molecules that may contribute directly to development of juvenile or maturity-onset diabetes. Two new concepts are proposed: First, the receptor for insulin occupies a locus on the plasma membrane coupled to Mg++-activated (Na+ + K+ )- ATPase; hormone activation stimulates ATPase activity. As a result of the activation of the membrane ATPase enzyme system by insulin, concentrations of K+ and Mg + + increase at critical intracellular loci; these ions then serve as "second messengers." The second new" concept is that the genetic abnormality in human diabetes mellitus lies in a single or multiple enzyme defect that leads to accumulation of a fragment from growth hormone. One such fragment is known to inhibit glucose uptake and fatty acid synthesis. DIABETES 21 (Suppl. 2).
Diet-induced physiological changes in the liver that promote improved blood glucose control. Insulin initiates its action by binding to a glycoprotein receptor on the surface of the cell. This receptor consists of an alpha-subunit, which binds the hormone, and a beta-subunit, which is an insulin-stimulated, tyrosine-specific protein kinase. Activation of this kinase is believed to generate a signal that eventually results in insulin's action on glucose, lipid, and protein metabolism. The growth-promoting effects of insulin appear to occur through activation of receptors for the family of related insulin-like growth factors. Both genetic and acquired abnormalities in the number of insulin receptors, the activity of the receptor kinase, and the various post-receptor steps in insulin action occur in disease states leading to tissue resistance to insulin action. It stimulates 1.Transport of glucose ions, Amino acids, 2.Glycogen formation, 3.Glucose conversion to triglycerides, 4.Nucleicacid synthesis and protein synthesis.
Diabetes Type 1, It occurs in approximately 10 percent of all cases, is an autoimmune disease in which the immune system, by mistake, attacks its own insulin-producing cells so that insufficient amounts of insulin are produced - or no insulin at all. Type 1 affects predominantly young people and usually makes its debut before the age of 30, and most frequently between the ages of 10 and 14. Rest 90% are type II;
In the early 1920s, Frederick Banting, John Macleod, George Best and Bertram Collip isolated the hormone insulin and purified it so that it could be administered to humans. This was a major breakthrough in the treatment of diabetes type 1.
Symptoms of type 1 diabetes; Excessive thirst and dehydration, frequent urination, hunger, accompanied by weight loss; blurred vision; weakness, tiredness, or sleepiness; vomiting or nausea, back foot foot breaks ; sudden irritability.
Diabetes Type 1, which occurs in approximately 10 percent of all cases, is an autoimmune disease in which the immune system, by mistake, attacks its own insulin-producing cells so that insufficient amounts of insulin are produced - or no insulin at all. Type 1 affects predominantly young people and usually makes its debut before the age of 30, and most frequently between the ages of 10 and 14. Rest 90% are type II;
In the early 1920s, Frederick Banting, John Macleod, George Best and Bertram Collip isolated the hormone insulin and purified it so that it could be administered to humans. This was a major breakthrough in the treatment of diabetes type 1.
Insulin is produced in the pancreas. To be more specific, it's produced by the beta cells in the islets of Langerhans in the pancreas. . If sugar level becomes too low, it can result in a coma and eventually death. Give orange juice or inject insulin; If the levels are low, the patient suffers from an overdose of insulin. https://www.nobelprize.org

https://www.ncbi.nlm.nih.gov

https://www.shutterstock.com
The islets of Langerhans are responsible for the endocrine function of the pancreas. Each islet contains beta, alpha, and delta cells that are responsible for the secretion of hormones.
Insulin is produced in the pancreas. To be more specific, it's produced by the beta cells in the islets of Langerhans in the pancreas. . If sugar level becomes too low, it can result in a coma and eventually death. Give orange juice or inject insulin; If the levels are low, the patient suffers from an overdose of insulin; the person can go into coma. https://www.nobelprize.org
Type 2:
Type 2 diabetes begins with insulin resistance. This means that the cells don't react to insulin the way they are supposed to do. Diabetes 2 will be 400 million or more in the entire population USA. https://www.nobelprize.org/
· fatigue, excessive thirst, frequent urination; blurred vision; mood changes; a high rate of infections; slow healing process.
· Insulin resistance, compensatory hyperinsulnamia; compensatory hyperinsulinaemia occurs when pancreatic β cell secretion increases to maintain normal blood glucose levels in the setting of peripheral insulin resistance in muscle and adipose tissue; Insulin resistance syndrome, Metabolic syndrome;
Insulin protein consists of two polypeptide chains A and B. The A chain comprises 21 amino acids and the B chain 30 amino acids. The A chain has an N-terminal helix linked to an anti-parallel C-terminal helix; the B chain has a central helical segment. The two chains are joined by 2 disulphide bonds, which join the N- and C-terminal helices of the A chain to the central helix of the B chain. In pro-insulin, a connecting peptide links the N-terminus of the A chain to the C-terminus of the B chain. If the levels are low, the patient suffers from an overdose of insulin. https://www.nobelprize.org; Insulin resistance, compensatory hyperinsulnamia; compensatory hyperinsulinaemia occurs when pancreatic β cell secretion increases to maintain normal blood glucose levels in the setting of peripheral insulin resistance in muscle and adipose tissue; Insulin resistance syndrome, Metabolic syndrome; Type 2 diabetes begins with insulin resistance. This means that the cells don't react to insulin the way they are supposed to do. Insulin resistance, compensatory hyperinsulnamia; ompensatory hyperinsulinaemia occurs when pancreatic β cell secretion increases to maintain normal blood glucose levels in the setting of peripheral insulin resistance in muscle and adipose tissue; Insulin resistance syndrome, Metabolic syndrome;
The A chain comprises 21 amino acids and the B chain 30 amino acids. The A chain has an N-terminal helix linked to an anti-parallel C-terminal helix; the B chain has a central helical segment. The two chains are joined by 2 disulphide bonds, which join the N- and C-terminal helices of the A chain to the central helix of the B chain. In pro-insulin, a connecting peptide links the N-terminus of the A chain to the C-terminus of the B chain.
Mechanisms of Insulin Secretion;
Increased levels of glucose induce the “first phase” of glucose-mediated insulin secretion by release of insulin from secretory granules in the β cell. Glucose entry into the β cell is sensed by glucokinase, which phosphorylates glucose to glucose-6-phosphate (G6P), generating ATP.12 Closure of K+-ATP-dependent channels results in membrane depolarization and activation of voltage dependent calcium channels leading to an increase in intracellular calcium concentration; this triggers pulsatile insulin secretion.13Augmentation of this response occurs by both a K+-ATP channel-independent Ca2+-dependent pathway and K+-ATP channel-independent Ca2+-independent pathways of glucose action.10 Other mediators of insulin release include activation of phospholipases and protein kinase C (e.g. by acetycholine) and by stimulation of adenylyl cyclase activity and activation of β cell protein kinase A, which potentiates insulin secretion. This latter mechanism may be activated by hormones, such as vasoactive intestinal peptide (VIP), PACAP, GLP-1, and GIP. These factors appear to play a significant role in the second phase of glucose mediated insulin secretion, after refilling of secretory granules translocated from reserve pools.

Insulin elicits a ROS-activated and an IP3-dependent Ca2+release, which both impinge on GLUT4 translocation http://jcs.biologists.org/conten; Ariel Contreras-Ferrat et al.

http://www.medicinehack.com/
Insulin binds to receptor on target sites on the surface of hepatocyte cells, muscle cells and brain cells. These sites have an intrinsic tyrosine kinase activity that leads to receptor auto phosphorylation and recruitment of intracellular signaling molecules. The latter results is widespread metabolic and mitogenic effects of insulin as shown in the diagram above.
Nutrients in the GI tract stimulate the secretion of hormones known as incretins which amplify glucose-induced insulin release. These account for the greater insulin response to oral, as opposed to intravenous, glucose. GIP and GLP-1 are the two most important incretin hormones. GLP-1 also inhibits glucagon release, delays gastric emptying and reduces appetite. https://www.ncbi.nlm.nih.gov
The classical symptoms of diabetes are polyuria (frequent urination), polydipsia (increased thirst) and polyphagia (increased hunger).
The receptor for insulin consists of an alpha-subunit, which binds the hormone, and a beta-subunit, which is an insulin-stimulated, tyrosine-specific protein kinase. Activation of this kinase is believed to generate a signal that eventually results in insulin's action on glucose, lipid, and protein metabolism. The growth-promoting effects of insulin appear to occur through activation of receptors for the family of related insulin-like growth factors. Both genetic and acquired abnormalities in the number of insulin receptors, the activity of the receptor kinase, and the various post-receptor steps in insulin action occur in disease states leading to tissue resistance to insulin action.
Nutrients in the GI tract stimulate the secretion of hormones known as incretins which amplify glucose-induced insulin release. These account for the greater insulin response to oral, as opposed to intravenous, glucose. GIP and GLP-1 are the two most important incretin hormones. GLP-1 also inhibits glucagon release, delays gastric emptying and reduces appetite. https://www.ncbi.nlm.nih.gov

Pancreatic beta cells produce insulin, which is a hexamer; https://en.wikipedia.org
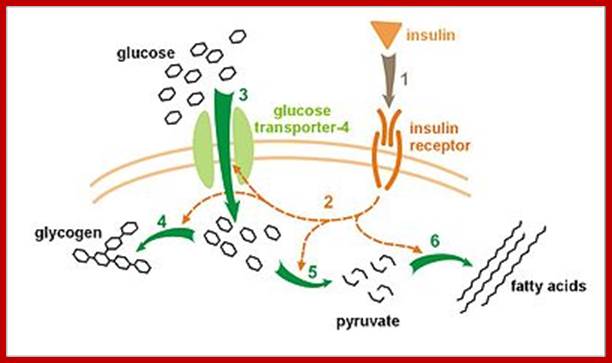
Effect of insulin on glucose uptake and metabolism: Insulin binds to its receptor (1), which starts many protein activation cascades (2). These include translocation of Glut-4 transporter to the plasma membrane and influx of glucose (3), glycogen synthesis (4), glycolysis (5) and triglyceride synthesis (6).
Increased potassium uptake – forces cells synthesizing glycogen (a very spongy, "wet" substance, that increases the content of intracellular water, and its accompanying K+ions)[56] to absorb potassium from the extracellular fluids; lack of insulin inhibits absorption. Insulin's increase in cellular potassium uptake lowers potassium levels in blood plasma. This possibly occurs via insulin-induced translocation of the Na+/K+-ATPase to the surface of skeletal muscle cells.
Once an insulin molecule has docked onto the receptor and effected its action, it may be released back into the extracellular environment, or it may be degraded by the cell. The two primary sites for insulin clearance are the liver and the kidney. The liver clears most insulin during first-pass transit, whereas the kidney clears most of the insulin in systemic circulation. Degradation normally involves endocytosis of the insulin-receptor complex, followed by the action of insulin-degrading enzyme. An insulin molecule produced endogenously by the pancreatic beta cells is estimated to be degraded within about one hour after its initial release into circulation ((insulin half-life ~ 4–6 minutes).
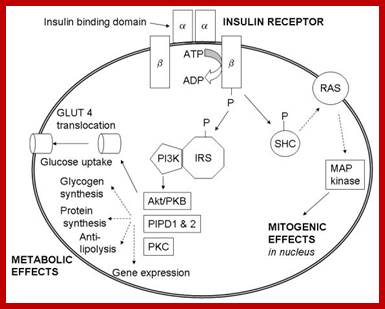
NCBi.nlm,nih.gov https://www.ncbi.nlm.nih.gov
What causes insulin resistance and Diabetes; Insulin resistance and high levels of insulin and lipids all precede the development of metabolic dysfunction. Which metabolic factor is to blame?
Type 2 diabetes is a metabolic disease categorized, primarily, by reduced insulin sensitivity, β-cell dysfunction, and elevated hepatic glucose production [1]. Insulin resistance is widely accepted as the starting point for the progression from glucose intolerance to overt type 2 diabetes. Therefore understanding the underlining mechanisms of insulin resistance pathophysiology is of great importance to the development of novel and effective treatments.
The notion that 5' AMP-activated protein kinase (AMPK) mediates the anti-hyperglycaemic action of metformin has recently been challenged by genetic loss-of-function studies, thrusting the AMPK-independent effects of the drug into the spotlight for the first time in more than a decade. Key AMPK-independent effects of the drug include the mitochondrial actions that have been known for many years and which are still thought to be the primary site of action of metformin. Coupled with recent evidence of AMPK-independent effects on the counter-regulatory hormone glucagon, new paradigms of AMPK-independent drug action are beginning to take shape. In this review we summarise the recent research developments on the molecular action of metformin.

Alan R. Saltiel & C. Ronald Kahn http://www.nature.com/nature
![]()
http://journal.frontiersin.org/article/
The insulin receptor is a tyrosine kinase that undergoes auto phosphorylation, and catalyzes the phosphorylation of cellular proteins such as members of the IRS family, Shc and Cbl. Upon tyrosine phosphorylation, these proteins interact with signaling molecules through their SH2 domains, resulting in a diverse series of signaling pathways, including activation of PI(3)K and downstream PtdIns(3,4,5)P3-dependent protein kinases, ras and the MAP kinase cascade, and Cbl/CAP and the activation of TC10. These pathways act in a concerted fashion to coordinate the regulation of vesicle trafficking, protein synthesis, enzyme activation and inactivation, and gene expression, which results in the regulation of glucose, lipid and protein metabolism.
The pathogenesis of insulin resistance, a major component of type 2 diabetes, has been the subject of extensive study, much of which has focused on signaling events or mitochondrial and endoplasmic reticulum dysfunction. A large body of data converges to suggest that insulin resistance also involves transcriptional and epigenomic (i.e., nuclear) events. Several nuclear receptors regulate insulin sensitivity in both positive and negative ways, including peroxisome proliferator-activated receptor γ (PPARγ) and the glucocorticoid receptor (GR). Insulin sensitivity can be affected by many transcription factors, cofactors, and chromatin-modifying enzymes working coordinately in different tissues and cell types.
Diabetic cardiomyopathy (DCM); Diabetic cardiomyopathy (DCM)
Cardiac metab; http://journal.frontiersin.org/article/ Insulin-stimulated glucose uptake in cardiomyocytes is mediated primarily through mobilization of glucose transporter 4 (GLUT4). Basal cardiac glucose uptake is mediated by glucose transporter 1 (GLUT1), however, contraction-mediated activation of GLUT4 translocation may also contribute significantly to myocardial glucose uptake (Abel, 2004). We have reported that GLUT4-mediated glucose uptake in cardiomyocytes can be activated through a G protein, phosphatidylinositol 3-kinase (PI3K)γ and 1,4,5-inositol-triphosphate (IP3) receptor (IP3R) signaling axis, leading to generation of a cytoplasmic Ca+2 signal that mobilizes GLUT4
Insulin resistance in most cases is believed to be manifest at the cellular level via post-receptor defects in insulin signaling; genetic polymorphisms of tyrosine phosphorylation of the insulin receptor, IRS proteins or PIP-3 kinase, or may involve abnormalities of GLUT 4 function.
Those tissues defined as insulin dependent, based on intracellular glucose transport, are principally adipose tissue and muscle. However, insulin’s actions are pleotropic and widespread, as are the manifestations of insulin resistance and the associated compensatory hyperinsulinemia.
Transport in muscle is via GLUT4, 60% of insulin is taken by muscle cells. In the fed state insulin promotes glycogen synthesis via activation of glycogen synthase. This enables energy to be released anaerobically via glycolysis, e.g. during intense muscular activity. Adipose tissue; Intracellular glucose transport into adipocytes in the postprandial state is insulin-dependent via GLUT 4;
Since lipoprotein lipase activity is insulin-dependent and impaired by insulin resistance, peripheral uptake of triglycerides from VLDL is also diminished. These mechanisms contribute to the observed hypertriglyceridemia of insulin resistance. Mitogenic effects of insulin (and growth hormone) are mediated via hepatic production of insulin-like growth factors and potentially via suppression of sex-hormone binding globulin (SHBG) production.
Insulin and its actions play an important role in various aspects of endothelial function, e.g. nitric oxide production, while insulin resistance is strongly associated with endothelial dysfunction. While the brain is not insulin-dependent so far as intracellular glucose uptake is concerned, insulin receptors have been located in the brain and are concentrated in the olfactory bulb, hypothalamus, hippocampus,40 retina and vessels of the choroid plexus, as well as in regions of the striatum and cerebral cortex e.g. medial temporal lobes. Insulin is believed to act as a neuropeptide, involved in satiety, appetite regulation, olfaction, memory and cognition. https://www.ncbi.nlm.nih.gov-
The molecular mechanism of insulin action.Kahn CR.
Insulin initiates its action by binding to a glycoprotein receptor on the surface of the cell. This receptor consists of an alpha-subunit, which binds the hormone, and a beta-subunit, which is an insulin-stimulated, tyrosine-specific protein kinase. Activation of this kinase is believed to generate a signal that eventually results in insulin's action on glucose, lipid, and protein metabolism. Activation of beta subunits lead to GLUT4 to be transported to the cellular PM. This leads to the transport of glucose from external into cells thus creates reduction of sugar in extra cellular fluids. The growth-promoting effects of insulin appear to occur through activation of receptors for the family of related insulin-like growth factors. Both genetic and acquired abnormalities in the number of insulin receptors, the activity of the receptor kinase, and the various post-receptor steps in insulin action occur in disease states leading to tissue resistance to insulin action. https://www.ncbi.nlm.nih.gov, www.slideshare.net
![]()
www.slideshare.net?top figure
The work is focused on the action of thiazolidinedione (TZDs), oral anti-diabetic drugs which reduce insulin resistance and exert insulin-sensitizing action directly on tissues. According to the professor Carme Caelles, at her laboratory in the Barcelona Science Park, "the action mechanism of TZDs is not well known yet. Their receptor (the PPARγ) has been identified; but we do not know yet how they act at molecular level.

Insulin Polypeptide chain
![]()
Proinsulin consists of three domains: an amino-terminal B chain, a carboxy-terminal A chain and a connecting peptide in the middle known as the C peptide. Within the endoplasmic reticulum, proinsulin
Comparison of gene expression -Of 6,451 genes surveyed, transcriptional patterns of 85 genes showed alterations in the diabetic patients after withdrawal of treatment, when compared with patterns in the nondiabetic control subject; Insulin treatment reduced the difference in patterns between diabetic and nondiabetic control subjects (improved) in all but 11 gene transcripts, which included genes involved in structural and contractile functions, growth and tissue development, stress response, and energy metabolism.
These improved transcripts included genes involved in insulin signaling, transcription factors, and mitochondrial maintenance. However, insulin treatment altered the transcription of 29 additional genes involved in signal transduction; structural and contractile functions; growth and tissue development; and protein, fat, and energy metabolism. Type 2 diabetic patients had elevated circulating insulin during the insulin-treated phase, although their blood glucose levels (98.8 ± 6.4 vs. 90.0 ± 2.9 mg/dl for diabetic vs. control) were similar to those of the control subjects. In contrast, after withdrawal of treatment, the diabetic patients had reduced SI and elevated blood glucose (224.0 ± 26.2 mg/dl), although their insulin levels were similar to those of the nondiabetic control subjects. This study identified several candidate genes for muscle insulin resistance, complications associated with poor glycemic control, and effects of insulin treatment in people with type 2 diabetes.
Insulin inhibits breakdown of fat in adipose tissue by inhibiting the intracellular lipase that hydrolyzes triglycerides to release fatty acids. Insulin also increases the permeability of many cells to potassium, magnesium and phosphate ions. The effect on potassium is clinically important. Insulin activates sodium-potassium ATPases in many cells, causing a flux of potassium into cells. Under certain circumstances, injection of insulin can kill patients because of its ability to acutely suppress plasma potassium concentrations.
In the early 1920s, Frederick Banting, John Macleod, George Best and Bertram Collip isolated the hormone insulin and purified it so that it could be administered to humans. This was a major breakthrough in the treatment of diabetes type 1. Insulin is produced in the pancreas. To be more specific, it's produced by the beta cells in the islets of Langerhans in the pancreas. . If sugar level becomes too low, it can result in a coma and eventually death. Give oranghe juice or inject insulin; If the levels are low, the patient suffers from an overdose of insulin. https://www.nobelprize.org
Mechanisms of Insulin Secretion
Increased levels of glucose induce the “first phase” of glucose-mediated insulin secretion by release of insulin from secretory granules in the β cell. Glucose entry into the β cell is sensed by glucokinase, which phosphorylates glucose to glucose-6-phosphate (G6P), generating ATP?. Closure of K+-ATP-dependent channels results in membrane depolarization and activation of voltage dependent calcium channels leading to an increase in intracellular calcium concentration; this triggers pulsatile insulin secretion.13Augmentation of this response occurs by both a K+-ATP channel-independent Ca2+-dependent pathway and K+-ATP channel-independent Ca2+-independent pathways of glucose action.10 Other mediators of insulin release include activation of phospholipases and protein kinase C (e.g. by acetycholine) and by stimulation of adenylyl cyclase activity and activation of β cell protein kinase A, which potentiates insulin secretion. This latter mechanism may be activated by hormones, such as vasoactive intestinal peptide (VIP), PACAP, GLP-1, and GIP. These factors appear to play a significant role in the second phase of glucose mediated insulin secretion, after refilling of secretory granules translocated from reserve pools
Nutrients in the GI tract stimulate the secretion of hormones known as incretins which amplify glucose-induced insulin release. These account for the greater insulin response to oral, as opposed to intravenous, glucose. GIP and GLP-1 are the two most important incretin hormones. GLP-1 also inhibits glucagon release, delays gastric emptying and reduces appetite. https://www.ncbi.nlm.nih.gov.
Type 2 diabetes is a metabolic disease categorized, primarily, by reduced insulin sensitivity, β-cell dysfunction, and elevated hepatic glucose production [1]. Insulin resistance is widely accepted as the starting point for the progression from glucose intolerance to overt type 2 diabetes. Therefore understanding the underlining mechanisms of insulin resistance pathophysiology is of great importance to the development of novel and effective treatments.
The notion that 5' AMP-activated protein kinase (AMPK) mediates the anti-hyperglycaemic action of metformin has recently been challenged by genetic loss-of-function studies, thrusting the AMPK-independent effects of the drug into the spotlight for the first time in more than a decade. Key AMPK-independent effects of the drug include the mitochondrial actions that have been known for many years and which are still thought to be the primary site of action of metformin. Coupled with recent evidence of AMPK-independent effects on the counter-regulatory hormone glucagon, new paradigms of AMPK-independent drug action are beginning to take shape. In this review we summarise the recent research developments on the molecular action of metformin.
The insulin receptor is a tyrosine kinase that undergoes autophosphorylation, and catalyses the phosphorylation of cellular proteins such as members of the IRS family, Shc and Cbl. Upon tyrosine phosphorylation, these proteins interact with signalling molecules through their SH2 domains, resulting in a diverse series of signalling pathways, including activation of PI(3)K and downstream PtdIns(3,4,5)P3-dependent protein kinases, ras and the MAP kinase cascade, and Cbl/CAP and the activation of TC10. These pathways act in a concerted fashion to coordinate the regulation of vesicle trafficking, protein synthesis, enzyme activation and inactivation, and gene expression, which results in the regulation of glucose, lipid and protein metabolism. Alan R. Saltiel & C. Ronald Kahn http://www.nature.com/nature . The pathogenesis of insulin resistance, a major component of type 2 diabetes, has been the subject of extensive study, much of which has focused on signaling events or mitochondrial and endoplasmic reticulum dysfunction. A large body of data converges to suggest that insulin resistance also involves transcriptional and epigenomic (i.e., nuclear) events. Several nuclear receptors regulate insulin sensitivity in both positive and negative ways, including peroxisome proliferator-activated receptor γ (PPARγ) and the glucocorticoid receptor (GR). Insulin sensitivity can be affected by many transcription factors, cofactors, and chromatin-modifying enzymes working coordinately in different tissues and cell types. Diabetic cardiomyopathy (DCM); Diabetic cardiomyopathy (DCM).
Cardiac metab; Insulin-stimulated glucose uptake in cardiomyocytes is mediated primarily through mobilization of glucose transporter 4 (GLUT4). Basal cardiac glucose uptake is mediated by glucose transporter 1 (GLUT1), however, contraction-mediated activation of GLUT4 translocation may also contribute significantly to myocardial glucose uptake (Abel, 2004)
We have reported that GLUT4-mediated glucose uptake in cardiomyocytes can be activated through a G protein, phosphatidylinositol 3-kinase (PI3K)γ and 1,4,5-inositol-triphosphate (IP3) receptor (IP3R) signaling axis, leading to generation of a cytoplasmic Ca+2 signal that mobilizes GLUT4
Insulin resistance in most cases is believed to be manifest at the cellular level via post-receptor defects in insulin signaling; genetic polymorphisms of tyrosine phosphorylation of the insulin receptor, IRS proteins or PIP-3 kinase, or may involve abnormalities of GLUT 4 function.
Those tissues defined as insulin dependent, based on intracellular glucose transport, are principally adipose tissue and muscle. However, insulin’s actions are pleotropic and widespread, as are the manifestations of insulin resistance and the associated compensatory hyperinsulinaemia.
Transport in muscle is via GLUT4, 60% of insulin is Tken by muscle cells. n the fed state insulin promotes glycogen synthesis via activation of glycogen synthase. This enables energy to be released anaerobically via glycolysis, e.g. during intense muscular activity.
Adipose tissue; Intracellular glucose transport into adipocytes in the postprandial state is insulin-dependent via GLUT 4;
since lipoprotein lipase activity is insulin-dependent and impaired by insulin resistance, peripheral uptake of triglycerides from VLDL is also diminished. These mechanisms contribute to the observed hypertriglyceridaemia of insulin resistance.
Mitogenic effects of insulin (and growth hormone) are mediated via hepatic production of insulin-like growth factors and potentially via suppression of sex-hormone binding globulin (SHBG) production.
Insulin and its actions play an important role in various aspects of endothelial function, e.g. nitric oxide production, while insulin resistance is strongly associated with endothelial dysfunction.
While the brain is not insulin-dependent so far as intracellular glucose uptake is concerned, insulin receptors have been located in the brain and are concentrated in the olfactory bulb, hypothalamus, hippocampus,40 retina and vessels of the choroid plexus, as well as in regions of the striatum and cerebral cortex e.g. medial temporal lobes. Insulin is believed to act as a neuropeptide, involved in satiety, appetite regulation, olfaction, memory and cognition.
The molecular mechanism of insulin action. Kahn CR.
Insulin initiates its action by binding to a glycoprotein receptor on the surface of the cell. This receptor consists of an alpha-subunit, which binds the hormone, and a beta-subunit, which is an insulin-stimulated, tyrosine-specific protein kinase. Activation of this kinase is believed to generate a signal that eventually results in insulin's action on glucose, lipid, and protein metabolism. The growth-promoting effects of insulin appear to occur through activation of receptors for the family of related insulin-like growth factors. Both genetic and acquired abnormalities in the number of insulin receptors, the activity of the receptor kinase, and the various post-receptor steps in insulin action occur in disease states leading to tissue resistance to insulin action. https://www.ncbi.nlm.nih.gov. www.slideshare.net.

Insulin protein-monomer and hexamer
![]()
Insulin receptor- Alpa and Beta subunits bound to cell membrane
The insulin receptor is a tyrosine kinase. In other words, it functions as an enzyme that transfers phosphate groups from ATP to tyrosine residues on intracellular target proteins. Binding of insulin to the alpha subunits causes the beta subunits to phosphorylate themselves (autophosphorylation), thus activating the catalytic activity of the receptor. The activated receptor then phosphorylates a number of intracellular proteins, which in turn alters their activity, thereby generating a biological response. Several intracellular proteins have been identified as phosphorylation substrates for the insulin receptor, the best-studied of which is insulin receptor substrate 1 or IRS-1. When IRS-1 is activated by phosphorylation, a lot of things happen. Among other things, IRS-1 serves as a type of docking center for recruitment and activation of other enzymes that ultimately mediate insulin's effects. A more detailed look at these processes is presented in the section on insulin transduction.
Insulin facilitates entry of glucose into muscle, adipose and several other tissue-via GLUT4, Insulin stimulates the liver to store glucose in the form of glycogen. A well-known effect of insulin is to decrease the concentration of glucose in blood, Insulin promotes synthesis of fatty acids in the liver.
The work is focused on the action of thiazolidinediones (TZDs), oral anti-diabetic drugs which reduce insulin resistance and exert insulin-sensitizing action directly on tissues. According to the professor Carme Caelles, at her laboratory in the Barcelona Science Park, "the action mechanism of TZDs is not well known yet. Their receptor (the PPARγ) has been identified; but we do not know yet how they act at molecular level.
Insulin also increases the permiability of many cells to potassium, magnesium and phosphate ions. The effect on potassium is clinically important. Insulin activates sodium-potassium ATPases in many cells, causing a flux of potassium into cells. Under certain circumstances, injection of insulin can kill patients because of its ability to acutely suppress potassium Plasma concentrations.
Proinsulin consists of three domains: an amino-terminal B chain, a carboxy-terminal A chain and a connecting peptide in the middle known as the C peptide. Within the endoplasmic reticulum, proinsulin
Comparison of gene expression -Of 6,451 genes surveyed, transcriptional patterns of 85 genes showed alterations in the diabetic patients after withdrawal of treatment, when compared with patterns in the nondiabetic control subject; Insulin treatment reduced the difference in patterns between diabetic and nondiabetic control subjects (improved) in all but 11 gene transcripts, which included genes involved in structural and contractile functions, growth and tissue development, stress response, and energy metabolism.
These improved transcripts included genes involved in insulin signaling, transcription factors, and mitochondrial maintenance. However, insulin treatment altered the transcription of 29 additional genes involved in signal transduction; structural and contractile functions; growth and tissue development; and protein, fat, and energy metabolism. Type 2 diabetic patients had elevated circulating insulin during the insulin-treated phase, although their blood glucose levels (98.8 ± 6.4 vs. 90.0 ± 2.9 mg/dl for diabetic vs. control) were similar to those of the control subjects. In contrast, after withdrawal of treatment, the diabetic patients had reduced SI and elevated blood glucose (224.0 ± 26.2 mg/dl), although their insulin levels were similar to those of the nondiabetic control subjects. This study identified several candidate genes for muscle insulin resistance, complications associated with poor glycemic control, and effects of insulin treatment in people with type 2 diabetes.

http://care.diabetesjournals.org/ Harold E. Lebovitz, hlebovitz1@hotmail.com.
In type 2 diabetes, the abnormalities in insulin secretion are multiple. One of the initial defects is a loss of the early phase of meal-stimulated insulin secretion. This is followed by an inability of the β-cell to increase insulin secretion sufficient to overcome hepatic and peripheral insulin resistance. Type 2 diabetes is characterized by a progressive decrease in both β-cell mass and secretory function so that, in most individuals, absolute insulin deficiency occurs in the late stages of the disease.
In normal physiology, β-cell insulin secretion is coupled immediately with changes in the plasma glucose level. The secretory response is rapid (within a minute or two), and because the half-life of insulin is ~5 min, there is little lag time in the glucose regulatory system. Endogenously secreted insulin goes via the portal vein to the liver, where 30–80% of it is either metabolized or used. The portal vein-to-peripheral arterial insulin ratio is ~2:1. The administration of insulin exogenously eliminates the rapid regulation of plasma glucose, since the insulin must be taken up slowly and without regulation from the subcutaneous injection site. The kinetics are determined by the nature of the injected insulin formulation. Additionally, as illustrated in the figure, it is necessary to create hyperinsulinemia in the periphery to achieve adequate insulin in the liver (portal-to-peripheral insulin levels ~1:2 to appropriately regulate hepatic glucose production and/or glucose uptake.
Administration of exogenous insulin provides a different insulin gradient than that occurring after endogenous insulin secretion. Endogenous insulin secretion acts initially on the liver where a major portion of it is taken up and <50% reaches the peripheral tissues. Exogenously administered insulin must circulate through the peripheral tissues before it can reach the liver; therefore, peripheral hyperinsulinemia is necessary to attain adequate insulin to regulate the liver.

http://care.diabetesjournals.org/ Harold E.
Lebovitz, hlebovitz1@hotmail.com. In type 2 diabetes, the abnormalities in insulin secretion are multiple. One of the initial defects is a loss of the early phase of meal-stimulated insulin secretion. This is followed by an inability of the β-cell to increase insulin secretion sufficient to overcome hepatic and peripheral insulin resistance. Type 2 diabetes is characterized by a progressive decrease in both β-cell mass and secretory function so that, in most individuals, absolute insulin deficiency occurs in the late stages of the disease.
1. In normal physiology, β-cell insulin secretion is coupled immediately with changes in the plasma glucose level (1). The secretory response is rapid (within a minute or two), and because the half-life of insulin is ~5 min, there is little lag time in the glucose regulatory system. Endogenously secreted insulin goes via the portal vein to the liver, where 30–80% of it is either metabolized or used (2). The portal vein-to-peripheral arterial insulin ratio is ~2:1. The administration of insulin exogenously eliminates the rapid regulation of plasma glucose, since the insulin must be taken up slowly and without regulation from the subcutaneous injection site. The kinetics are determined by the nature of the injected insulin formulation. Additionally, as illustrated in Fig. 1, it is necessary to create hyperinsulinemia in the periphery to achieve adequate insulin in the liver (portal-to-peripheral insulin levels ~1:2) to appropriately regulate hepatic glucose production and/or glucose uptake.
Administration of exogenous insulin provides a different insulin gradient than that occurring after endogenous insulin secretion. Endogenous insulin secretion acts initially on the liver where a major portion of it is taken up and <50% reaches the peripheral tissues. Exogenously administered insulin must circulate through the peripheral tissues before it can reach the liver; therefore, peripheral hyperinsulinemia is necessary to attain adequate insulin to regulate the liver.
Metformin;
Metformin Glucophage is basically a refined herbal medicine derived from a flower called French lilac (Galega officinalis) (goat’s rue or French lilac). “Glucophage” (glucose eater) is for T2D type 2 diabetes. Metformin is derived from Galega officinalis, an herb described to treat several ailments since the seventeenth century. Studies in the early 1900s showed guanidine, a compound isolated from the herb, was able to lower blood sugar in animal models. These models also suggested guanidine by itself was likely too potent and toxic to use clinically in humans.
The effectiveness of metformin for treatment of type 2 diabetes is due to its multiple mechanisms of action. Metformin works by decreasing the amount of sugar made by the liver and consequently decreasing the secretion of insulin from the pancreas. Metformin also slows the absorption of sugar in the intestine and increases insulin sensitivity in the muscles and periphery to help the body utilize sugar. Since metformin does not increase the amount of insulin secreted from the pancreas, as seen with other oral anti-diabetic medications such as sulfonylureas, it is does not cause weight gain or excessively reduce blood sugar. - See more at: http://www.rxeconsult.com/
Evidence from large clinical studies such as the UKPDS (United Kingdom Prospective Diabetes Study) shows early treatment with metformin in type 2-diabetes reduces long-term cardiovascular complications that can lead to death. This clinical finding may be attributed to metformin’s benefits on fat (lipids), oxidative stress, and inflammation. Metformin has shown positive effects on lowering triglycerides, lowering “bad” cholesterol (LDL), and slightly increasing “good” cholesterol (HDL). Additionally, recent mechanistic studies have shown how metformin can have anti-inflammatory effects on the vascular wall and improve lipid metabolism in macrophages (cells that help fight infection in the body). Other in vitro studies are evaluating the mechanism for metformin reducing oxidative stress. Results suggest several possible pathways for reducing reactive oxygen species, including decreases in specific proteins involved in intracellular metabolism. - See more at:
Treatment for prevention and treatment of solid tumor cancers (breast, prostate, colorectal, and endometrial). Numerous in vitro and studies are attempting to explain the mechanism for how metformin may inhibit tumor growth. - See more at: http://www.rxeconsult.com/healthcare-articles
Metformin: The notion that 5' AMP-activated protein kinase (AMPK) mediates the anti-hyperglycaemic action of metformin has recently been challenged by genetic loss-of-function studies, thrusting the AMPK-independent effects of the drug into the spotlight for the first time in more than a decade. Key AMPK-independent effects of the drug include the mitochondrial actions that have been known for many years and which are still thought to be the primary site of action of metformin. Coupled with recent evidence of AMPK-independent effects on the counter-regulatory hormone glucagon, new paradigms of AMPK-independent drug action are beginning to take shape. In this review we summarise the recent research developments on the molecular action of metformin. Graham Rena; https://www.ncbi.nlm.nih.gov
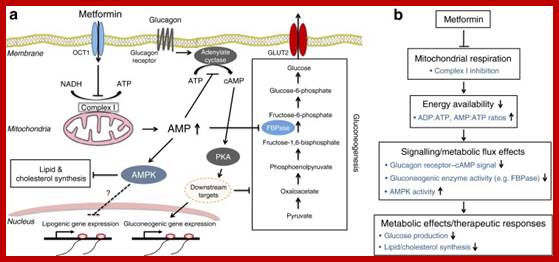
(a, b) Schematic diagram of the anti-hyperglycaemic action of metformin on the liver cell. Part (b) shows a simplified version of (a). Metformin is transported into hepatocytes mainly via OCT1, resulting in an inhibition of the mitochondrial respiratory chain (complex I) through a currently unknown mechanism(s). The resulting deficit in energy production is balanced by reducing the consumption of energy in the cell, particularly reduced gluconeogenesis in the liver. This is mediated in two main ways. First, a decrease in ATP and a concomitant increase in AMP concentration occur, which is thought to contribute to the inhibition of gluconeogenesis directly (because of the energy/ATP deficit). Second, increased AMP levels function as a key signalling mediator that has been proposed to (1) allosterically inhibit cAMP–PKA signalling through suppression of adenylate cyclase, (2) allosterically inhibit FBPase, a key gluconeogenic enzyme, and (3) activates AMPK. This leads to inhibition of gluconeogenesis (1 and 2) and lipid/cholesterol synthesis (3), which may contribute to the longer term metabolic and therapeutic responses to the drug. FBPase; fructose-1,6-bisphosphatase
Metformin and Alzheimer’s disease:
Metformin; (other names -Glucophage, Glucophage XR, Glumetza, Riomet); Nobody could explain how metformin helped, but then Canadian researchers showed that metformin reduces cell mutations and DNA damage. Spanish scientists published in the journal Cell Cycle that metformin in two ways. Like a chemotherapy drug, it blocks certain enzymes cancer cells need to reproduce. But the scientists wrote that metformin’s glucose-lowering and insulin-lowering effects may be more important. Metformin mimics the effect of severely restricting calories. The lowered insulin level enables healthy cells to reproduce better, so they don’t become cancerous. As a powerful antioxidant, metformin may actually slow aging. Anti-aging guru Ward Dean, MD, calls metformin “the most effective anti-aging drug there is. Mostly, through a mild and transient inhibition of the mitochondrial respiratory-chain complex 1. Metformin also activates the AMP-activated protein kinase (AMPK). Acutely decrease hepatic glucose production, mostly through a mild and transient inhibition of the mitochondrial respiratory chain complex I.
Although the molecular target of metformin has been elusive for several years, Zhou et al. reported that the activation of AMPK (AMP-activated protein kinase) was intimately associated with the pleiotropic actions of metformin. AMPK is a phylogenetically conserved serine/threonine protein kinase viewed as a fuel gauge monitoring systemic and cellular energy status, and which plays a crucial role in protecting cellular functions under energy-restricted conditions. AMPK is a heterotrimeric protein consisting of a catalytic α-subunit and two regulatory subunits, β and γ, and each subunits have at least two isoforms. AMPK is activated by an increase in the intracellular AMP/ATP ratio resulting from an imbalance between ATP production and consumption. Activation of AMPK involves AMP binding to regulatory sites on the γ-subunits. This causes conformational changes that allosterically activate the enzyme and inhibit dephosphorylation of Thr within the activation loop of the catalytic α-subunit. AMPK activation requires phosphorylation of Thr by upstream kinases, identified as the tumour suppressor STK11 (serine/threonine kinase 11)/LKB1 and CaMKKβ (Ca2+/calmodulin-dependent protein kinase β), which is stimulated further by the allosteric activator AMP. Moreover, it has been recently shown that ADP, and therefore the ADP/ATP ratio, could also play a regulatory role on AMPK by binding to specific domains in the γ-subunit . Activated AMPK switches cells from an anabolic to a catabolic state, shutting down the ATP-consuming synthetic pathways and restoring energy balance. This regulation involves phosphorylation by AMPK of key metabolic enzymes and transcription factors/co-activators modulating gene expression. As a result, glucose, lipid and protein synthesis, as well as cell growth, are inhibited, whereas fatty acid oxidation and glucose uptake are stimulated.
In addition, the resulting decrease in hepatic energy status activates AMPK (AMP-activated protein kinase), a cellular metabolic sensor, providing a generally accepted mechanism for the action of metformin on hepatic gluconeogenesis.
Owing to its high acid dissociation constant (pKa=12.4), metformin exists in a positively charged protonated form under physiological conditions and, as a result, can only partially cross the plasma membrane by passive- diffusion. Thus its intracellular transport is mediated by different isoforms of OCTs depending on the tissue under consideration (e.g. OCT1 in the liver or OCT2 in the kidney). Once inside the cytosolic compartment, mitochondria then constitute the primary target of metformin. The positive charge of metformin has been proposed to account for its accumulation within the matrix of energized mitochondria, driven by the membrane potential, whereas the apolar hydrocarbon side chain of the drug could also promote binding to hydrophobic structures, especially the phospholipids of mitochondrial membranes. Although the exact mechanism(s) by which metformin acts at the molecular level remains unknown, it has been shown that the drug inhibits the mitochondrial respiratory chain specifically at the level of complex I without affecting any other steps in the mitochondrial machinery. This unique property of the drug induces a decrease in NADH oxidation, proton pumping across the inner mitochondrial membrane and oxygen consumption rate, leading to lowering of the proton gradient and, ultimately, to a reduction in proton-driven synthesis of ATP from ADP and Pi.
The demonstration that the respiratory-chain complex 1, a potent antihyperglycemic agent now recommended as the first line oral therapy for type 2 diabetes (T2D). It is to acutely decrease hepatic glucose production, mostly through a mild and transient inhibition of the mitochondrial respiratory-chain complex 1. In addition, the resulting decrease in hepatic energy status activates the AMP-activated protein kinase (AMPK), a cellular metabolic sensor, providing a generally accepted mechanism for metformin action on hepatic gluconeogenic program. The demonstration that the respiratory-chain complex 1, but not AMPK, is the primary target of metformin was recently strengthened by showing that the metabolic effect of the drug is preserved in liver-specific AMPK-deficient mice. Metformin restore ovarian function in polycystic ovary syndrome, reduce fatty liver and to lower microvascular and macrovascular complications associated with T2D. Its use was also recently suggested as an adjuvant treatment for cancer or gestational diabetes, and for the prevention in pre-diabetic populations.
Once treated with metformin, however, CBP was activated to the levels of nondiabetic mice, and their blood glucose levels returned to normal. However, when given to diabetic mice with defective copies of CBP, metformin had no effect on blood glucose levels, a proof that metformin works through CBP. As a cellular energy sensor, AMP-activated protein kinase (AMPK) is activated in response to a variety of conditions that deplete cellular energy levels, such as nutrient starvation (especially glucose), hypoxia and exposure to toxins that inhibit the mitochondrial respiratory chain complex;
Metformin and Alzheimer’s disease
Metformin (brand name Glucophage, Glucophage XR, Glumetza, Riomet) ,Nobody could explain how metformin helped, but then Canadian researchers showed that metformin reduces cell mutations and DNA damage.
Spanish scientists published in the journal Cell Cycle that metformin seems to block cancer in two ways. Like a chemotherapy drug, it blocks certain enzymes cancer cells need to reproduce. But the scientists wrote that metformin’s glucose-lowering and insulin-lowering effects may be more important. Metformin mimics the effect of severely restricting calories. The lowered insulin level enables healthy cells to reproduce better, so they don’t become cancerous.
As a powerful antioxidant, metformin may actually slow aging. Anti-aging guru Ward Dean, MD, calls metformin “the most effective anti-aging drug there is mostly through a mild and transient inhibition of the mitochondrial respiratory-chain complex 1, Activates the AMP-activated protein kinase (AMPK),
Acutely decrease hepatic glucose production, mostly through a mild and transient inhibition of the mitochondrial respiratory chain complex I; Although the molecular target of metformin has been elusive for several years, Zhou et al. reported that the activation of AMPK (AMP-activated protein kinase) was intimately associated with the pleiotropic actions of metformin. AMPK is a phylogenetically conserved serine/threonine protein kinase viewed as a fuel gauge monitoring systemic and cellular energy status, and which plays a crucial role in protecting cellular functions under energy-restricted conditions. AMPK is a heterotrimeric protein consisting of a catalytic α-subunit and two regulatory subunits, β and γ, and each subunit has at least two isoforms. AMPK is activated by an increase in the intracellular AMP/ATP ratio resulting from an imbalance between ATP production and consumption. Activation of AMPK involves AMP binding to regulatory sites on the γ-subunits. This causes conformational changes that allosterically activate the enzyme and inhibit dephosphorylation of Thr172 within the activation loop of the catalytic α-subunit. AMPK activation requires phosphorylation on Thr172 by upstream kinases, identified as the tumour suppressor STK11 (serine/threonine kinase 11)/LKB1 and CaMKKβ (Ca2+/calmodulin-dependent protein kinase β), which is stimulated further by the allosteric activator AMP. Moreover, it has been recently shown that ADP, and therefore the ADP/ATP ratio, could also play a regulatory role on AMPK by binding to specific domains in the γ-subunit [19,20]. Activated AMPK switches cells from an anabolic to a catabolic state, shutting down the ATP-consuming synthetic pathways and restoring energy balance. This regulation involves phosphorylation by AMPK of key metabolic enzymes and transcription factors/co-activators modulating gene expression [18]. As a result, glucose, lipid and protein synthesis, as well as cell growth, are inhibited, whereas fatty acid oxidation and glucose uptake are stimulated.
In addition, the resulting decrease in hepatic energy status activates AMPK (AMP-activated protein kinase), a cellular metabolic sensor, providing a generally accepted mechanism for the action of metformin on hepatic gluconeogenesis.
https://www.ncbi.nlm.nih.gov The mitochondrial respiratory chain complex 1 is the primary target of metformin
Owing to its high acid dissociation constant (pKa=12.4), metformin exists in a positively charged protonated form under physiological conditions and, as a result, can only partially cross the plasma membrane by passive diffusion. Thus its intracellular transport is mediated by different isoforms of OCTs depending on the tissue under consideration (e.g. OCT1 in the liver or OCT2 in the kidney). Once inside the cytosolic compartment, mitochondria then constitute the primary target of metformin. The positive charge of metformin has been proposed to account for its accumulation within the matrix of energized mitochondria, driven by the membrane potential, whereas the apolar hydrocarbon side chain of the drug could also promote binding to hydrophobic structures, especially the phospholipids of mitochondrial membranes. Although the exact mechanism(s) by which metformin acts at the molecular level remains unknown, it has been shown that the drug inhibits the mitochondrial respiratory chain specifically at the level of complex I without affecting any other steps in the mitochondrial machinery. This unique property of the drug induces a decrease in NADH oxidation, proton pumping across the inner mitochondrial membrane and oxygen consumption rate, leading to lowering of the proton gradient and, ultimately, to a reduction in proton-driven synthesis of ATP from ADP and Pi.
Restores ovarian function in polycystic ovary syndrome, reduce fatty liver and to lower microvascular and macrovascular complications associated with T2D, Its use was also recently suggested as an adjuvant treatment for cancer or gestational diabetes, and for the prevention in pre-diabetic populations.
Once treated with metformin, however, CBP was activated to the levels of nondiabetic mice, and their blood glucose levels returned to normal. However, when given to diabetic mice with defective copies of CBP, metformin had no effect on blood glucose levels, a proof that metformin works through CBP. As a cellular energy sensor, AMP-activated protein kinase (AMPK) is activated in response to a variety of conditions that deplete cellular energy levels, such as nutrient starvation (especially glucose), hypoxia and exposure to toxins that inhibit the mitochondrial respiratory chain complex;
Acutely decreases hepatic glucose production, mostly through a mild and transient inhibition of the mitochondrial respiratory-chain complex 1. In addition, the resulting decrease in hepatic energy status activates the AMP-activated protein kinase (AMPK), a cellular metabolic sensor, providing a generally accepted mechanism for metformin action on hepatic gluconeogenic program. The demonstration that the respiratory-chain complex 1, but not AMPK, is the primary target of metformin was recently strengthened by showing that the metabolic effect of the drug is preserved in liver-specific AMPK-deficient mice.
AMPK activation by metformin is not a result of direct activation; instead, metformin inhibits complex I of the mitochondrial respiratory chain, leading to an increased AMP:ATP ratio. The main effect of this drug from the biguanide family is to acutely decrease hepatic glucose production, mostly through a mild and transient inhibition of the mitochondrial respiratory-chain complex 1. In addition, the resulting decrease in hepatic energy status activates the AMP-activated protein kinase (AMPK), a cellular metabolic sensor, providing a generally accepted mechanism for metformin action on hepatic gluconeogenic program. The demonstration that the respiratory-chain complex 1, but not AMPK, is the primary target of metformin was recently strengthened by showing that the metabolic effect of the drug is preserved in liver-specific AMPK-deficient mice. https://www.ncbi.nlm.nih.gov/
The mitochondrial respiratory-chain complex 1 is the primary target of metformin:
Due to its high acid dissociation constant (pKa=12.4) metformin exists in a positively charged protonated form under physiological conditions and, as a result, can only marginally cross the plasma membrane by passive diffusion. Thus, its intracellular transport is mediated by different isoforms of the organic cation transporters (OCT) depending of the tissue considered (e.g. OCT1 in liver or OCT2 in kidney). Once inside the cytosolic compartment, mitochondria then constitute the primary target of metformin. The positive charge of metformin was proposed to account for its accumulation within the matrix of energized mitochondria, driven by the membrane potential (Δϕ), whereas the apolar hydrocarbon side-chain of the drug could also promote binding to hydrophobic structures, especially the phospholipids of mitochondrial membranes [31]. Although the exact mechanism(s) by which metformin acts at the molecular level remains unknown, it has been shown that the drug inhibits mitochondrial respiratory-chain specifically at the complex 1 level without affecting any other steps of the mitochondrial machinery. This unique property of the drug induces a decrease in NADH oxidation, proton pumping across the inner mitochondrial membrane and oxygen consumption rate, leading to lowering of the proton gradient (Δϕ) and ultimately to a reduction in proton-driven synthesis of ATP from ADP and inorganic phosphate (Pi).

http://cancerpreventionresearch.aacrjournals.org
https://bmcbiol.biomedcentral.com/

Rosina Pryor, Filipe Cabreiro;http://www.biochemj.org
Potential molecular mechanisms of metformin action on hepatic gluconeogenesis;
After hepatic uptake through OCT1, the mitochondria is the primary target of metformin which exerts specific and AMPK-independent inhibition of respiratory-chain complex 1. The resultant mild decrease in energy status leads to acute and transient inhibition of energy-consuming gluconeogenic pathway. In addition, through AMPK-dependent and -independent regulatory points, metformin can lead to the inhibition of glucose production by disrupting gluconeogenesis gene expression. In parallel, the LKB1-dependent activation of AMPK triggered by ATP depletion could reduce hepatic lipogenesis and exert an indirect effect on hepatic insulin sensitivity to control hepatic glucose output.
Glipizide acts by partially blocking potassium channels among beta cells of pancreatic islets of Langerhans. By blocking potassium channels, the cell depolarizes which results in the opening of voltage-gated calcium channels. The resulting calcium influx encourages insulin release from beta cells Wikipedia; Glipizide acts by partially blocking potassium channels among beta cells of pancreatic islets of Langerhans. By blocking potassium channels, the cell depolarizes which results in the opening of voltage-gated calcium channels. The resulting calcium influx encourages insulin release from beta cells. Acts on Cardiovascular system, Nephrons, Metformin’s action on cancer, anti-age.
Metformin and vildaglipitin- good for Dibetes-2; Vildaglipitin primarily by enhancing pancreatic (α and β) islet function. Improves Isulin secretion,
The main effect of this drug from the biguanide family is to acutely decrease hepatic glucose production, mostly through a mild and transient inhibition of the mitochondrial respiratory-chain complex 1. decrease in hepatic energy status activates the AMP-activated protein kinase (AMPK), a cellular metabolic sensor, providing a generally accepted mechanism for metformin action on hepatic gluconeogenic program. The main effect of this drug from the biguanide family is to acutely decrease hepatic glucose production, mostly through a mild and transient inhibition of the mitochondrial respiratory-chain complex 1.
Metformin decreases in hepatic energy status, which activates the AMP-activated protein kinase (AMPK), a cellular metabolic sensor, providing a generally accepted mechanism for metformin action on hepatic gluconeogenic program. In addition, the resulting decrease in hepatic energy status activates the AMP-activated protein kinase (AMPK), a cellular metabolic sensor, providing a generally accepted mechanism for metformin action on hepatic gluconeogenic program.
may also have therapeutic potential in other conditions including diabetic nephropathy, cardiovascular diseases, polycystic ovary disease and the prevention or treatment of cancer. Metformin is also frequently described as an insulin sensitizer leading to reduction in insulin resistance and significant reduction of plasma fasting insulin level. The improvement in insulin sensitivity by metformin could be ascribed to its positive effects on insulin receptor expression and tyrosine kinase activity ;
The preferential action of metformin in hepatocytes is due to the predominant expression of the organic cation transporter 1 (OCT1), which has been shown to facilitate cellular uptake of metformin .
Hou et al. reported that the activation of AMP-activated protein kinase (AMPK) was intimately associated with the pleiotropic actions of metformin. AMPK is a phylogenetically conserved serine/threonine protein kinase viewed as a fuel gauge monitoring systemic and cellular energy status and which plays a crucial role in protecting cellular functions under energy-restricted conditions. AMPK is a heterotrimeric protein consisting of a catalytic α-subunit and two regulatory subunits β and γ and each subunit has at least two isoforms. AMPK is activated by increase in the intracellular AMP-on-ATP ratio resulting from imbalance between ATP production and consumption. Activation of AMPK involves AMP binding to regulatory sites on the γ subunits. This causes conformational changes that allosterically activate the enzyme and inhibit dephosphorylation of Thr172 within the activation loop of the catalytic α subunit. AMPK activation requires phosphorylation on Thr172 by upstream kinases, identified as the tumor suppressor serine/threonine kinase 11 (STK11/LKB1) and CaMKKβ, which is further stimulated by the allosteric activator AMP . Moreover, it has been recently shown that ADP, and therefore the ADP-on-ATP ratio, could also play a regulatory role on AMPK by binding to specific domains on the γ subunit [19, 20]. Activated AMPK switches cells from an anabolic to a catabolic state, shutting down the ATP-consuming synthetic pathways and restoring energy balance. This regulation involves phosphorylation by AMPK of key metabolic enzymes and transcription factors/co-activators modulating gene expression [18]. As a result, glucose, lipid and protein synthesis as well as cell growth are inhibited whereas fatty acid oxidation and glucose uptake are stimulated. https://www.ncbi.nlm.nih.gov
Potential molecular mechanisms of metformin action on hepatic gluconeogenesis;
After hepatic uptake through OCT1, the mitochondria is the primary target of metformin which exerts specific and AMPK-independent inhibition of respiratory-chain complex 1. The resultant mild decrease in energy status leads to acute and transient inhibition of energy-consuming gluconeogenic pathway. In addition, through AMPK-dependent and -independent regulatory points, metformin can lead to the inhibition of glucose production by disrupting gluconeogenesis gene expression. In parallel, the LKB1-dependent activation of AMPK triggered by ATP depletion could reduce hepatic lipogenesis and exert an indirect effect on hepatic insulin sensitivity to control hepatic glucose output. https://www.ncbi.nlm.nih.gov; It also exerts an inhibitory effect on mitochondrial ROS production by selectively blocking the reverse electron flow through the respiratory-chain complex 1.
Empagliflozin 10 and 25 mg for 24 weeks as add-on to metformin therapy significantly improved glycemic control, weight, and BP, and were well-tolerated. http://care.diabetesjournals.org/ Metformin mainly acts by reducing hepatic glucose production through inhibition of gluconeogenesis and may increase glucose uptake in peripheral tissue.
The kidney has emerged as a therapeutic target in the treatment of type 2 diabetes. Approximately 90% of glucose filtered by the kidney is reabsorbed by the sodium glucose cotransporter 2 (SGLT2), located in the proximal tubule of the nephron. SGLT2 inhibitors are novel oral anti-diabetes agents that, by reducing glucose reabsorption, increase urinary glucose excretion and reduce hyperglycemia independent of β-cell function and insulin resistance . This mechanism carries a low risk of hypoglycemia, with additional benefits of weight loss and reductions in blood pressure (BP).
And in the latest analysis, also reported earlier this month at the ADA meeting, empagliflozin was shown to be renoprotective too, significantly reducing the incidence of worsening nephropathy, by 39%. Check for glycated Hemoglobin(A1C), https://www.ncbi.nlm.nih.gov. Metformin’s action on cancer, anti-age; Acts on Cardiovascular system, Nephrons,
Empagliflozin is an orally active, potent, selective inhibitor of SGLT2. In phase II trials, treatment with empagliflozin as an add-on to metformin therapy was shown to be well-tolerated, with low rates of hypoglycemia, and to lead to significant reductions in HbA1c and body weight that were maintained over 90 weeks; Empagliflozin is an orally active, potent, selective inhibitor of SGLT2. In phase II trials, treatment with empagliflozin as an add-on to metformin therapy was shown to be well-tolerated, with low rates of hypoglycemia, and to lead to significant reductions in HbA1c and body weight that were maintained over 90 weeks;
And in the latest analysis, also reported earlier this month at the ADA meeting, empagliflozin was shown to be renoprotective too, significantly reducing the incidence of worsening nephropathy, by 39
Metformin and vildaglipitin- good for Dibetes-2; Vildaglipitin primarily by enhancing pancreatic (α and β) islet function. Improves Insulin secretion,
The main effect of this drug from the biguanide family is to acutely decrease hepatic glucose production, mostly through a mild and transient inhibition of the mitochondrial respiratory-chain complex 1. decrease in hepatic energy status activates the AMP-activated protein kinase (AMPK), a cellular metabolic sensor, providing a generally accepted mechanism for metformin action on hepatic gluconeogenic program. The main effect of this drug from the biguanide family is to acutely decrease hepatic glucose production, mostly through a mild and transient inhibition of the mitochondrial respiratory-chain complex 1.
Metformin decreases in hepatic energy status, which activates the AMP-activated protein kinase (AMPK), a cellular metabolic sensor, providing a generally accepted mechanism for metformin action on hepatic gluconeogenic program. In addition, the resulting decrease in hepatic energy status activates the AMP-activated protein kinase (AMPK), a cellular metabolic sensor, providing a generally accepted mechanism for metformin action on hepatic gluconeogenic program.
It may also have therapeutic potential in other conditions including diabetic nephropathy, cardiovascular diseases, polycystic ovary disease and the prevention or treatment of cancer. Metformin is also frequently described as an insulin sensitizer leading to reduction in insulin resistance and significant reduction of plasma fasting insulin level. The improvement in insulin sensitivity by metformin could be ascribed to its positive effects on insulin receptor expression and tyrosine kinase activity ;
The preferential action of metformin in hepatocytes is due to the predominant expression of the organic cation transporter 1 (OCT1), which has been shown to facilitate cellular uptake of metformin . It is
reported that the activation of AMP-activated protein kinase (AMPK) was intimately associated with the pleiotropic actions of metformin. AMPK is a phylogenetically conserved serine/threonine protein kinase viewed as a fuel gauge monitoring systemic and cellular energy status and which plays a crucial role in protecting cellular functions under energy-restricted conditions. AMPK is a heterotrimeric protein consisting of a catalytic α-subunit and two regulatory subunits β and γ and each subunit has at least two isoforms. AMPK is activated by increase in the intracellular AMP-on-ATP ratio resulting from imbalance between ATP production and consumption. Activation of AMPK involves AMP binding to regulatory sites on the γ subunits. This causes conformational changes that allosterically activate the enzyme and inhibit dephosphorylation of Thr172 within the activation loop of the catalytic α subunit. AMPK activation requires phosphorylation on Thr172 by upstream kinases, identified as the tumor suppressor serine/threonine kinase 11 (STK11/LKB1) and CaMKKβ, which is further stimulated by the allosteric activator AMP. Moreover, it has been recently shown that ADP, and therefore the ADP-on-ATP ratio, could also play a regulatory role on AMPK by binding to specific domains on the γ subunit. Activated AMPK switches cells from an anabolic to a catabolic state, shutting down the ATP-consuming synthetic pathways and restoring energy balance. This regulation involves phosphorylation by AMPK of key metabolic enzymes and transcription factors/co-activators modulating gene expression [18]. As a result, glucose, lipid and protein synthesis as well as cell growth are inhibited whereas fatty acid oxidation and glucose uptake are stimulated. https://www.ncbi.nlm.nih.gov
TB drug- Bedaquiline; http://www.tbfacts.org/
Bedaquiline works by blocking an enzyme inside the Mycobacterium tuberculosis bacteria called ATP synthase. This enzyme is used by the bacteria to generate energy. Without the ability to generate energy, the TB bacteria die and the patient’s condition can start to improve. - See more at main TB drugs isoniazid (INH) and rifampicin (RMP). This means that the drugs don’t work. - See more at: http://www.tbfacts.org/. Ethionamide, kanamycin, pyrazinamide, Afloxacin, cycloserine/terizidone are available alternatives. See more at: costing US$30,000 in the United States; Bedaquiline also called Sirturo.
Cancer:
Despite current successes in hematological cancers, we are only in the beginning of exploring the powerful potential of CAR redirected T cells in the control and elimination of resistant, metastatic, or recurrent non hematological cancers. This review discusses the application of the CAR T cell therapy, its challenges, and strategies for successful clinical and commercial translation.
CARs are genetically engineered receptors that combine the specific binding domains from a tumor targeting antibody with T cell signaling domains to allow specifically targeted antibody redirected T cell activation.
Using immune system to treat cancer.;
CARs are genetically engineered receptors that combine the specific binding domains from a tumor targeting antibody with T cell signaling domains to allow specifically targeted antibody redirected T cell activation.
Despite current successes in hematological cancers, we are only in the beginning of exploring the powerful potential of CAR redirected T cells in the control and elimination of resistant, metastatic, or recurrent nonhematological cancers. This review discusses the application of the CAR T cell therapy, its challenges, and strategies for successful clinical and commercial translation. https://www.cancer.gov
Cancer Cell; Immune Checkpoint Modulators, Immune Cell Therapy, Therapeutic Antibodies, Cancer Treatment Vaccines, Immune System Modulators, Research at NCI, https://www.cancer.gov;
The immune system’s natural capacity to detect and destroy abnormal cells may prevent the development of many cancers. However, cancer cells are sometimes able to avoid detection and destruction by the immune system. Cancer cells may:
- reduce the expression of tumor antigens on their surface, making it harder for the immune system to detect them
- express proteins on their surface that induce immune cell inactivation
- induce cells in the surrounding environment (microenvironment) to release substances that suppress immune responses and promote tumor cell proliferation and survival
· One immunotherapy approach is to block the ability of certain proteins, called immune checkpoint proteins, to limit the strength and duration of immune responses. These proteins normally keep immune responses in check by preventing overly intense responses that might damage normal cells as well as abnormal cells. But, researchers have learned that tumors can commandeer these proteins and use them to suppress immune responses.
· Blocking the activity of immune checkpoint proteins releases the "brakes" on the immune system, increasing its ability to destroy cancer cells.
· The first such drug to receive approval by FDA, ipilimumab (Yervoy®), for the treatment of advanced melanoma, blocks the activity of a checkpoint protein known as CTLA4, which is expressed on the surface of activated immune cells called cytotoxic T lymphocytes. CTLA4 acts as a "switch" to inactivate these T cells, thereby reducing the strength of immune responses; ipilimumab binds to CTLA4 and prevents it from sending its inhibitory signal.
Immunotherapy; Cancer;
Scanning electron micrograph of a human T lymphocyte (also called a T cell), as shown above, from the immune system of a healthy donor. Source: National Institute of Allergy and Infectious Diseases (NIAID).
· Therapy; Immune Checkpoint Modulators, Immune Cell Therapy, Therapeutic Antibodies, Cancer Treatment Vaccines, Immune System Modulators, Research at NCI,
The immune system’s natural capacity to detect and destroy abnormal cells may prevent the development of many cancers. However, cancer cells are sometimes able to avoid detection and destruction by the immune system. Cancer cells may:
- reduce the expression of tumor antigens on their surface, making it harder for the immune system to detect them
- express proteins on their surface that induce immune cell inactivation
- induce cells in the surrounding environment (microenvironment) to release substances that suppress immune responses and promote tumor cell proliferation and survival
· One immunotherapy approach is to block the ability of certain proteins, called immune checkpoint proteins, to limit the strength and duration of immune responses. These proteins normally keep immune responses in check by preventing overly intense responses that might damage normal cells as well as abnormal cells. But, researchers have learned that tumors can commandeer these proteins and use them to suppress immune responses.
· Blocking the activity of immune checkpoint proteins releases the "brakes" on the immune system, increasing its ability to destroy cancer cells.
· The first such drug to receive approval by FDA, ipilimumab (Yervoy®), for the treatment of advanced melanoma, blocks the activity of a checkpoint protein known as CTLA4, which is expressed on the surface of activated immune cells called cytotoxic T lymphocytes. CTLA4 acts as a "switch" to inactivate these T cells, thereby reducing the strength of immune responses; ipilimumab binds to CTLA4 and prevents it from sending its inhibitory signal.
Immune Cell Therapy
Progress is also being made with an experimental form of immunotherapy called adoptive cell transfer (ACT). In several small clinical trials testing ACT, some patients with very advanced cancer—primarily blood cancers—have had their disease completely eradicated. In some cases, these treatment responses have lasted for years.
In one form of ACT, T cells that have infiltrated a patient’s tumor, called tumor-infiltrating lymphocytes(TILs), are collected from samples of the tumor. TILs that show the greatest recognition of the patient's tumor cells in laboratory tests are selected, and large populations of these cells are grown in the laboratory. The cells are then activated by treatment with immune system signaling proteins called cytokines and infused into the patient’s bloodstream.
Another form of ACT that is being actively studied is CAR T-cell therapy. In this treatment approach, a patient’s T cells are collected from the blood and genetically modified to express a protein known as a chimeric antigen receptor, or CAR. Next, the modified cells are grown in the laboratory to produce large populations of the cells, which are then infused into the patient.
Therapeutic Antibodies; Therapeutic antibodies are antibodies made in the laboratory that are designed to cause the destruction of cancer cells. One class of therapeutic antibodies, called antibody–drug conjugates (ADCs), has proven to be particularly effective, with several ADCs having been approved by the FDA for the treatment of different cancers.
ADCs are created by chemically linking antibodies, or fragments of antibodies, to a toxic substance. The antibody portion of the ADC allows it to bind to a target molecule that is expressed on the surface of cancer cells. The toxic substance can be a poison, such as a bacterial toxin; a small-molecule drug; or a radioactive compound. Once an ADC binds to a cancer cell, it is taken up by the cell and the toxic substance kills the cell.
The FDA has approved several ADCs for the treatment of patients with cancer, including:
· ado-trastuzumab emtansine (Kadcyla®) for the treatment of some types of breast cancer
· brentuximab vedotin (Adcetris®) for Hodgkin lymphoma and a type of non-Hodgkin T-cell lymphoma
· ibritumomab tiuxetan (Zevalin®) for a type of non-Hodgkin B-cell lymphoma
rituximab (Rituxan, denileukin diftitox (ONTAK®)
Cancer Treatment Using Vaccines:
The use of cancer treatment (or therapeutic) vaccines is another approach to immunotherapy. These vaccines are usually made from a patient’s own tumor cells or from substances produced by tumor cells. They are designed to treat cancers that have already developed by strengthening the body’s natural defenses against the cancer.
In 2010, the FDA approved the first cancer treatment vaccine, sipuleucel-T (Provenge®), for use in some men with metastatic prostate cancer. Other therapeutic vaccines are being tested in clinical trials to treat a range of cancers, including brain, breast, and lung cancer.
Cancer immunotherapy via dendritic cells: Karolina Palucka1,2 & Jacques Banchereau3 Nature Reviews Cancer 12, 265-277 (April 2012)
Cancer immunotherapy attempts to harness the power and specificity of the immune system to treat tumours. The molecular identification of human cancer-specific antigens has allowed the development of antigen-specific immunotherapy. In one approach, autologous antigen-specific T cells are expanded ex vivo and then re-infused into patients. Another approach is through vaccination; that is, the provision of an antigen together with an adjuvant to elicit therapeutic T cells in vivo. Owing to their properties, dendritic cells (DCs) are often called 'nature's adjuvants' and thus have become the natural agents for antigen delivery. After four decades of research, it is now clear that DCs are at the Centre of the immune system owing to their ability to control both immune tolerance and immunity. Thus, DCs are an essential target in efforts to generate therapeutic immunity against cancer.
Despite current successes in hematological cancers, we are only in the beginning of exploring the powerful potential of CAR redirected T cells in the control and elimination of resistant, metastatic, or recurrent non-hematological cancers. This review discusses the application of the CAR T cell therapy, its challenges, and strategies for successful clinical and commercial translation.
Elements involved in TCR and CAR recognition and activation. The TCR is disulfide-linked heterodimer consisting of one α and one β chain expressed in complex with invariant CD3 chains (γ, δ, ζ, and ε). The TCR recognizes intracellular or extracellular proteins presented as peptides by MHC molecules. Costimulation of CD28 through its ligands, CD80/CD86, is required for optimal activation and production of interleukin-2 (IL-2) and other cytokines. While most hematological tumors express costimulatory molecules, solid tumor cells as well as antigen presenting cells in the tumor microenvironment usually lack such molecules. CARs recognize surface antigens in an MHC unrestricted manner. CARs are fusion proteins between single-chain variable fragments (scFv) from a monoclonal antibody and one or more T cell receptor intracellular signaling domains. Various hinges and transmembrane (TM) domains are used to link the recognition and the signaling molecules [5]. While first generation CARs signaled through the CD3ζ chain only, second generation CARs include a signaling domain from a costimulatory molecule, for example, CD28 (illustrated), 4-1BB, OX40, CD27, or ICOS.
Example of manufacturing and delivery pipeline of CAR T cell therapies: Peripheral blood mononuclear cells (PBMCs) are harvested from the patient (or a T cell donor) (a) and transferred to a good manufacturing practice (GMP) facility, where the T cells are isolated and activated in the presence of magnetic beads conjugated with CD3 and CD28 antibodies (b) and subsequently genetically engineered by viral transduction to express the CAR (c). The activated T cells are expanded ex vivo for a period, typically 10–14 days, to reach a therapeutic relevant number (d) before magnetic bead removal (e) and formulation, either for freezing or for adoptive transfer (f). The patient undergoes a conditional chemotherapy prior to infusion of the CAR T cells (g). https://www.ncbi.nlm.nih.gov

With sequence of a patient, in hand scientists can diagnose
genetic disease, identify future disease risk, home in on disease modifiers and
predict responses to drugs. sequence of a patient, scientists can diagnose
genetic disease, identify future disease risk, home in on disease modifiers and
predict responses to drugs.
http://www.moleculargenetics.utoronto.ca/
- Immune Check point Modulators and Immune Cell Therapy- white blood cell from the patients are used by gene modifications thus the T cells act on donors cancer cells and kill them.
- Active immunotherapy directs the immune system to attack tumor cells by targeting TAAs. Passive immunotherapies enhance existing anti-tumor responses and include the use of monoclonal antibodies, lymphocytes and cytokines. Those that bind to tumor antigens treat cancer. Cell surface receptors are common targets for antibody therapies and include CD20, CD274 and CD279. Once bound to a cancer antigen, antibodies can induce antibody-dependent cell-mediated cytotoxicity, activate the complement system, or prevent a receptor from interacting with its ligand, all of which can lead to
Cell death.
Approved antibodies include alemtuzumab, ipilimumab, nivolumab, ofatumumab and rituximab. https://www.technologyreview.com; Antonio Regalado, https://en.wikipedia.org.
Therapeutic Antibodies; https://www.ncbi.nlm.nih.gov; Masami Suzuki et al; Therapeutic antibodies: their mechanisms of action and the pathological findings they induce in toxicity studies; From the mid-1990’s, a series of therapeutic antibodies were launched that are now being used in clinic. The disease areas that therapeutic antibodies can target have subsequently expanded, and antibodies are currently utilized as pharmaceuticals for cancer, inflammatory disease, organ transplantation, cardiovascular disease, infection, respiratory disease, ophthalmologic disease, and so on. 1) the high level of specificity and affinity to the target molecule or antigen achieves a high level of efficacy and fewer adverse events, 2) their ability to target diverse molecules and the modes of action of the antibodies allow them to be applied to a wide range of therapeutic targets, and 3) modification and refinement by genetic engineering technology and the establishment of recombinant manufacturing technology has made industrial manufacturing possible. The efficacy of therapeutic antibodies stems from various natural functions of antibodies — neutralization, antibody-dependent cell-mediated cytotoxic (ADCC) activity, or complement-dependent cytotoxic (CDC) activity —, or the antibody can be utilized as a drug delivery carrier (missile therapy)
Mechanisms of action of therapeutic antibodies. https://www.ncbi.nlm.nih.gov
America’s biopharmaceutical research companies are using biological processes to develop 907 medicines and vaccines targeting more than 100 diseases. Many biologics are made from a variety of natural sources—human, animal or microorganisms. Like small-molecule drugs, some biologics are intended to treat diseases and medical conditions. Other biologics are used to prevent or diagnose disease. Examples of biological products include but are not limited to.
http://phrma-docs.phrma.org/ monoclonal antibodies • vaccines, including therapeutic vaccines • blood and blood products for transfusion and/or manufacturing into other products • gene therapies • cell therapies
The medicines discussed in this report are either in human clinical trials or under review by the U.S. Food and Drug Administration (FDA). These medicines often represent cutting-edge research in which the latest scientific discoveries are translated into novel therapies that provide new treatment options for patients. Increased understanding of the molecular and genetic bases of disease has opened up the development of a range of targeted treatments. For instance, monoclonal antibodies (mAbs) are proteins that help the immune system identify and bind to foreign substances. Thirty years after initial development, these therapies help treat some of the most costly and challenging diseases.
- https://www.cancer.gov; Cancer Treatment Vaccines; Cancer cells can carry both self-antigens as well as what are referred to as cancer-associated antigens. Cancer-associated antigens mark cancer cells as abnormal or foreign and can cause killer T cells to mount an attack against them. Cancer-associated antigens may be: Self antigens that are made in much larger amounts by cancer cells than normal cells and, thus, are viewed as foreign by the immune system
· Self -antigens that are not normally made by the tissue in which the cancer developed (for example, antigens that are normally made only by embryonic tissue but are expressed in an adult cancer) and, thus, are viewed as foreign by the immune system
· Newly formed antigens, or neo-antigens, that result from gene mutations in cancer cells and have not been seen previously by the immune system
· Vaccines are medicines that boost the immune system's natural ability to protect the body against “foreign invaders,” mainly infectious agents, that may cause disease. Cancer vaccines belong to a class of substances known as biological response modifiers. Biological response modifiers work by stimulating or restoring the immune system’s ability to fight infections and disease. There are two broad types of cancer vaccines: Preventive (or prophylactic) vaccines, which are intended to prevent cancer from developing in healthy people
· Treatment (or therapeutic) vaccines, which are intended to treat an existing cancer by strengthening the body’s natural immune response against the cancer. Treatment vaccines are a form of immunotherapy.
Two types of cancer preventive vaccines (human papillomavirus vaccines and hepatitis B virus vaccines) are available in the United States, and one treatment vaccine (for metastatic prostate cancer) is available.
The immune system’s natural capacity to detect and destroy abnormal cells may prevent the development of many cancers. However, cancer cells are sometimes able to avoid detection and destruction by the immune system. Cancer cells may: reduce the expression of tumor antigens on their surface, making it harder for the immune system to detect them; express proteins on their surface that induce immune cell inactivation, induce cells in the surrounding environment (microenvironment) to release substances that suppress immune responses and promote tumor cell proliferation and survival.
Immune check point Modulators:
One immunotherapy approach is to block the ability of certain proteins, called immune checkpoint proteins, to limit the strength and duration of immune responses. These proteins normally keep immune responses in check by preventing overly intense responses that might damage normal cells as well as abnormal cells. But, researchers have learned that tumors can commandeer these proteins and use them to suppress immune responses. Blocking the activity of immune checkpoint proteins releases the "brakes" on the immune system, increasing its ability to destroy cancer cells.
The first such drug to receive approval by FDA, ipilimumab (Yervoy®), for the treatment of advanced melanoma, blocks the activity of a checkpoint protein known as CTLA4, which is expressed on the surface of activated immune cells called cytotoxic T lymphocytes. CTLA4 acts as a "switch" to inactivate these T cells, thereby reducing the strength of immune responses; ipilimumab binds to CTLA4 and prevents it from sending its inhibitory signal.
Immune Cell Therapy;
Progress is also being made with an experimental form of immunotherapy called adoptive cell transfer (ACT). In several small clinical trials testing ACT, some patients with very advanced cancer—primarily blood cancers—have had their disease completely eradicated. In some cases, these treatment responses have lasted for years.
In one form of ACT, T cells that have infiltrated a patient’s tumor, called tumor-infiltrating lymphocytes(TILs), are collected from samples of the tumor. TILs that show the greatest recognition of the patient's tumor cells in laboratory tests are selected, and large populations of these cells are grown in the laboratory. The cells are then activated by treatment with immune system signaling proteins called cytokines and infused into the patient’s bloodstream.
Another form of ACT that is being actively studied is CAR T-cell therapy. In this treatment approach, a patient’s T cells are collected from the blood and genetically modified to express a protein known as a chimeric antigen receptor, or CAR. Next, the modified cells are grown in the laboratory to produce large populations of the cells, which are then infused into the patient.
Therapeutic Antibodies; Therapeutic antibodies are antibodies made in the laboratory that are designed to cause the destruction of cancer cells. One class of therapeutic antibodies, called antibody–drug conjugates (ADCs), has proven to be particularly effective, with several ADCs having been approved by the FDA for the treatment of different cancers. ADCs are created by chemically linking antibodies, or fragments of antibodies, to a toxic substance. The antibody portion of the ADC allows it to bind to a target molecule that is expressed on the surface of cancer cells. The toxic substance can be a poison, such as a bacterial toxin; a small-molecule drug; or a radioactive compound. Once an ADC binds to a cancer cell, it is taken up by the cell and the toxic substance kills the cell.
Cancer Treatment Vaccines
The use of cancer treatment (or therapeutic) vaccines is another approach to immunotherapy. These vaccines are usually made from a patient’s own tumor cells or from substances produced by tumor cells. They are designed to treat cancers that have already developed by strengthening the body’s natural defenses against the cancer.
In 2010, the FDA approved the first cancer treatment vaccine, sipuleucel-T (Provenge®), for use in some men with metastatic prostate cancer. Other therapeutic vaccines are being tested in clinical trials to treat a range of cancers, including brain, breast, and lung cancer.
Tumour immunology & immunotherapy;
Cancer immunotherapy via dendritic cells
Karolina Palucka1,2 & Jacques Banchereau3 Nature Reviews Cancer 12, 265-277 (April 2012)
Cancer immunotherapy attempts to harness the power and specificity of the immune system to treat tumours. The molecular identification of human cancer-specific antigens has allowed the development of antigen-specific immunotherapy. In one approach, autologous antigen-specific T cells are expanded ex vivo and then re-infused into patients. Another approach is through vaccination; that is, the provision of an antigen together with an adjuvant to elicit therapeutic T cells in vivo. Owing to their properties, dendritic cells (DCs) are often called 'nature's adjuvants' and thus have become the natural agents for antigen delivery. After four decades of research, it is now clear that DCs are at the Centre of the immune system owing to their ability to control both immune tolerance and immunity. Thus, DCs are an essential target in efforts to generate therapeutic immunity against cancer.
Despite current successes in hematological cancers, we are only in the beginning of exploring the powerful potential of CAR redirected T cells in the control and elimination of resistant, metastatic, or recurrent nonhematological cancers. This review discusses the application of the CAR T cell therapy, its challenges, and strategies for successful clinical and commercial translation.
Gene therapy: The first attempt at modifying human DNA was performed in 1980 by Martin Cline, but the first successful and approved nuclear gene transfer in humans was performed in May 1989; Between 1989 and February 2016, over 2,300 clinical trials had been conducted, more than half of them in phase I, The first attempt, an unsuccessful one, at gene therapy (as well as the first case of medical transfer of foreign genes into humans not counting organ transplantation) was performed by Martin Cline on 10 July 1980-for treating beta-thalassemia;
The first commercial gene therapy, Gendicine, was approved in China in 2003 for the treatment of certain cancers; In 2011 Neovasculgen was registered in Russia as the first-in-class gene-therapy drug for treatment of peripheral artery disease, including critical limb ischemia. In 2012 Glybera, a treatment for a rare inherited disorder, became the first treatment to be approved for clinical use in either Europe or the United States after its endorsement by the European Commission;
Somatic cell gene therapy - SCGT. Germline gene therapy GGT; A number of viruses have been used for human gene therapy, including retrovirus, adenovirus, lentivirus, herpes simplex, vaccinia and adeno-associated virus.
Antisense therapies; Cancer, Cytomegalovirus retinitis, familial hypercholesterol, Hemorrhagic fever viruses; HIV/AIDS, Spinal muscular atrophy, Duchenne muscular dystrophy, Hypertriglyceridemia, Hypertriglyceridemia, color blindness

http://learn.genetics.utah.edu/
Severe Combined Immune Deficiency (SCID)
http://learn.genetics.utah.edu/c-blindness
Advances in the field of epigenetics have allowed the design of new therapeutic strategies to address complex diseases such as type 1 diabetes (T1D). Clustered regularly interspaced short palindromic repeats (CRISPR)-on is a novel and powerful RNA-guided transcriptional activator system that can turn on specific gene expression; however, it remains unclear whether this system can be widely used or whether its use will be restricted depending on cell types, methylation promoter statuses or the capacity to modulate chromatin state. Our results revealed that the CRISPR-on system fused with transcriptional activators (dCas9-VP160) activated endogenous human INS, which is a silenced gene with a fully methylated promoter. Similarly, we observed a synergistic effect on gene activation when multiple single guide RNAs were used, and the transcriptional activation was maintained until day 21. Regarding the epigenetic profile, the targeted promoter gene did not exhibit alteration in its methylation status but rather exhibited altered levels of H3K9ac following treatment. Importantly, we showed that dCas9-VP160 acts on patients’ cells in vitro, particularly the fibroblasts of patients with T1D. CRISPR-on system for the activation of the endogenous human INS gene; http://www.nature.com/;, , , , and
Cancer cells;
Inherited; Genetics specialists estimate that only about 2 or 3 in every 100 cancers diagnosed (2 to 3%) are linked to an inherited gene fault. Pancreatin cancer is prevelant; Globally, 337,872 new pancreatic cancer cases and 330,391 deaths were estimated in 2012’; BRCA1, BRCA2, and p53 are examples of tumor suppressor genes, DNA repair genes; cell cycle regulatory genes,
Cell mediated therapy; Elizabeth J. Akins1 and Purnima Dubey
https://www.ncbi.nlm.nih.gov
The fairly recent use of molecular imaging techniques to track cell populations has presented researchers and clinicians with a powerful diagnostic tool for determining the efficacy of cell-mediated therapy for the treatment of cancer. ell mediated therapy /immunotherapy was found to be effective only in few cases. This review highlights the application of whole-body noninvasive radioisotopic, magnetic, and optical imaging methods for monitoring effector cells in vivo. Issues that affect sensitivity of detection, such as methods of cell marking, efficiency of cell labeling, toxicity, and limits of detection of imaging modalities, are discussed.
It is thought that the immune system regularly detects and destroys transformed cells, a process termed immune-surveillance ; Immunotherapy seeks to modulate immune function to target the cancerous cells that have evaded immune surveillance. Therefore, harnessing the power of the adaptive cellular immune system to eradicate tumors, while sparing normal tissues, is an attractive potential therapy for the treatment of cancer, particularly metastatic disease.
Major Effector cells used in cancer therapy include CD8+ cytotoxic T cells, CD4+ helper T cells, CD4+ CD25+ regulatory T cells, and natural killer cells CD8-CTLs, CD4 Thl,Th2, Regulatory T cells, NK1 (Naturla killer cells). Peptide fragments of antigens expressed by tumor cells are presented to the immune system by professional antigen-presenting cells (APCs), such as dendritic cells (DCs). As a result, cytolytic (CD8+) and helper (CD4+) T cells proliferate and release effector cell cytokines. When tumoricidal activity can be detected, cytolytic T cells (CTLs) are the primary effector cells, whereas CD4+ T helper cells provide help in the form of secreted cytokines. The generation of an effective antitumor immune response requires the concerted activity of all of these components of the immune system

“Best-case” scenario: CD8+ T cells are activated in draining lymph node (LN) by DCs expressing tumor antigen peptides on class I MHC (HLA-A, HLA-B, and HLA-C). Once activated, CD8+ T cells home to site of tumor, invading stroma to gain access to tumor cells. Antigen-expressing tumor cells are recognized and lysed by CD8+ T cells. HEV = high endothelial venule.
Therapeutic monoclonal antibodies target specific antigens found on the cell surface, such as transmembrane receptors or extracellular growth factors. In some cases, monoclonal antibodies are conjugated to radio-isotopes or toxins to allow specific delivery of these cytotoxic agents to the intended cancer cell target.
Small molecules can penetrate the cell membrane to interact with targets inside a cell. Small molecules are usually designed to interfere with the enzymatic activity of the target protein. For example, the small molecule STI-571 became known as imatinib (generic name) and is marketed by Novartis under the brand name Gleevec.
https://molcelltherapies.biomedcentral.com- read, it looks GOOOOD
Genetically Engineered Pharmaceuticals;
Factor VIII for males suffering from hemophilia A; Hemophilia A (HA, OMIM) is an X-linked bleeding disorder caused by heterogeneous mutations in the factor VIII gene (F8). The FVIII protein is required for propagation of the intrinsic coagulation pathway [1]. Hemophilia A, or congenital factor VIII deficiency, is the most common of the inherited bleeding disorders, its incidence is estimated to be between 1:5,000 and 1:10,000 in men http://www.sciencedirect.com/ Factor VIII (F8) is the only gene known to be associated with hemophilia A. F8 maps to the distal end of the long arm of the X-chromosome (Xq28) and spans 186 kb of genomic DNA. It consists of 26 exons that encode a 2351 amino acid precursor polypeptide. The mature FVIII protein consists of three homologous A domains, two homologous C domains and the unique B domain, which are arranged in the order A1-A2-B-A3-C1-C2 from the amino terminus to the carboxyl-terminal end. The different domains play an important role in the function of FVIII as each domain contains specific binding sites for different components of the clotting cascade and Genetic defects can affect these interaction sites and cause HA.
Factor IX; FactorX for hemophilia B- Hemophilia B is a rare genetic bleeding disorder in which affected individuals have insufficient levels of a blood protein called factor IX. Factor IX is a clotting factor. Clotting factors are specialized proteins needed for blood clotting, the process by which blood seals a wound to stop bleeding and promote healing. Individuals with hemophilia B do not bleed faster than unaffected individuals, they bleed longer. This is because they are missing a protein involved in blood clotting and are unable to effectively stop the flow of blood from a wound, injury or bleeding site. This is sometimes referred to as prolonged bleeding or a bleeding episode. The FDA approved the drug Idelvion, coagulation Factor IX (recombinant), albumin fusion protein in 2016. The drug is available for children and adults affected by Hemophilia B. Idelvion are manufactured by CSL Behring. https://rarediseases.org;
Ixinity, a recombinant factor IX product, was approved in 2015 for the control and prevention of bleeding episodes and for perioperative management in adults and children 12 years of age or older with hemophilia B. This medication is manufactured by Emergent BioSolutions, Inc.
Recombinant Factor IX: Recombinant factor IX products are manufactured in a laboratory. These genetically engineered products do not contain animal or human protein and are not derived from human blood; they are theoretically considered to be free of the risk of transmitting viruses. Recombinant factor IX therapy is the recommended treatment for individuals with hemophilia B. In the U.S., the currently available recombinant factor IX products are BeneFIX®, Rixubis®, and Alprolix®. https://rarediseases.org
- Human growth hormone (GH) Growth hormone (GH), also known as somatotropin (or as human growth hormone [hGH or HGH] in its human form), is a peptide hormone that stimulates growth, cell reproduction, and cell regeneration in humans and other animals, https://en.wikipedia.org/ A recombinant form of hGH called somatropin (INN) is used as a prescription drug to treat children's growth disorders and adult growth hormone deficiency. In the United States, it is only available legally from pharmacies, by prescription from a doctor also used to treat older persons.
Manufacturingdrugs; Genetically modified organism, Gene therapy https://en.wikipedia.org
Organisms are genetically engineered to discover the functions of certain genes. This could be the effect on the phenotype of the organism, where the gene is expressed or what other genes it interacts with. These experiments generally involve loss of function, gain of function, tracking and expression.
· Loss of function experiments, such as in a gene knockout experiment, in which an organism is engineered to lack the activity of one or more genes. A knockout experiment involves the creation and manipulation of a DNA construct in vitro, which, in a simple knockout, consists of a copy of the desired gene, which has been altered such that it is non-functional. Embryonic stem cells incorporate the altered gene, which replaces the already present functional copy. These stem cells are injected into blastocysts, which are implanted into surrogate mothers. This allows the experimenter to analyze the defects caused by this mutation and thereby determine the role of particular genes. It is used especially frequently in developmental biology. Another method, useful in organisms such as Drosophila (fruit fly), is to induce mutations in a large population and then screen the progeny for the desired mutation. A similar process can be used in both plants and prokaryotes. Loss of function tells whether or not a protein is required for a function, but it does not always mean it's sufficient, especially if a function requires multiple proteins and lose the said function if one protein is missing.
· Gain of function experiments, the logical counterpart of knockouts. These are sometimes performed in conjunction with knockout experiments to more finely establish the function of the desired gene. The process is much the same as that in knockout engineering, except that the construct is designed to increase the function of the gene, usually by providing extra copies of the gene or inducing synthesis of the protein more frequently. Gain of function is used to tell whether or not a protein is sufficient for a function, but it does not always mean it's required. Especially when dealing with genetic/functional redundancy.
· Tracking experiments, which seek to gain information about the localization and interaction of the desired protein. One way to do this is to replace the wild-type gene with a 'fusion' gene, which is a juxtaposition of the wild-type gene with a reporting element such as green fluorescent protein (GFP) that will allow easy visualization of the products of the genetic modification. While this is a useful technique, the manipulation can destroy the function of the gene, creating secondary effects and possibly calling into question the results of the experiment. More sophisticated techniques are now in development that can track protein products without mitigating their function, such as the addition of small sequences that will serve as binding motifs to monoclonal antibodies.
· Expression studies aim to discover where and when specific proteins are produced. In these experiments, the DNA sequence before the DNA that codes for a protein, known as a gene's promoter, is reintroduced into an organism with the protein coding region replaced by a reporter gene such as GFP or an enzyme that catalyzes the production of a dye. Thus the time and place where a particular protein is produced can be observed. Expression studies can be taken a step further by altering the promoter to find which pieces are crucial for the proper expression of the gene and are actually bound by transcription factor proteins; this process is known as promoter bashing.
One can manufacture mass quantities of the protein by growing the transformed organism in bioreactor equipment using techniques of industrial fermentation, and then purifying the protein.[94] Some genes do not work well in bacteria, so yeast, insect cells, or mammalians cells, each a eukaryote, can also be used. For example, in Alzheimer's disease, brain cells, or neurons, start to die off because of defective DNA. If doctors could grow new neurons from the patient's stem cells, they could replace the dying cells in the brain with cells engineered to have normal DNA, curing the disease. Currently, there is no cure for Alzheimer's and people's neurons slowly deteriorate over time, eventually making them unable to do even the most basic self-care. http://study.com/academy,
Some of the vast range of human substances being genetically made;
o Human epidermal growth factor (940); This gene encodes a member of the epidermal growth factor superfamily. The gene is located in chromosome 4. The encoded preproprotein is proteolytically processed to generate the 53-amino acid epidermal growth factor peptide. This protein acts a potent mitogenic factor that plays an important role in the growth, proliferation and differentiation of numerous cell types. This protein acts by binding with high affinity to the cell surface receptor, epidermal growth factor receptor. Defects in this gene are the cause of hypomagnesemia type 4. Dysregulation of this gene has been associated with the growth and progression of certain cancers. Alternative splicing results in multiple transcript variants, at least one of which encodes a preproprotein that is proteolytically processed. [provided by RefSeq, Jan 2016] https://www.ncbi.nlm.nih.gov
Epidermal growth factor (EGF) is a growth factor that stimulates cell growth, proliferation, and differentiation by binding to its receptor EGFR-6045 Da protein-with 53a.a.
EGF Family of proteins; Heparin-binding EGF-like growth factor (HB-EGF), transforming growth factor-α (TGF-α), Amphiregulin (AR), Epiregulin (EPR), Epigen, Betacellulin (BTC), neuregulin-1 (NRG1) neuregulin-2 (NRG2) neuregulin-3 (NRG3).neuregulin-4 (NRG4). Recombinant human epidermal growth factor, sold under the brand name Heberprot-P, is used to treat diabetic foot ulcers. This epidermal growth factor I cloned in plant soybean
5. Tissue plasminogen activator (980); Tissue plasminogen activator (abbreviated tPA or PLAT) is a protein involved in the breakdown of blood clots. It is a serine protease (EC 3.4.21.68) found on endothelial cells, the cells that line the blood vessels. As an enzyme, it catalyzes the conversion of plasminogen to plasmin, the major enzyme responsible for clot breakdown. Because, it works on the clotting system, tPA (such as alteplase, reteplase, and tenecteplase) is used in clinical medicine to treat embolic or thrombotic stroke. Use is contraindicated in hemorrhagic stroke and head trauma. The antidote for tPA in case of toxicity is aminocaproic acid. tPA may be manufactured using recombinant biotechnology techniques. tPA created this way may be referred to as recombinant tissue plasminogen activator (rtPA). It is sold as alteplase.
tPA is used in some cases of diseases that feature blood clots, such as pulmonary embolism, myocardial infarction, and stroke, in a medical treatment called thrombolysis. The most common use is for ischemic stroke. It can either be administered systemically, in the case of acute myocardial infarction, acute ischemic stroke, and most cases of acute massive pulmonary embolism, or administered through an arterial catheter directly to the site of occlusion in the case of peripheral arterial thrombi and thrombi in the proximal deep veins of the leg.[3] It has been suggested that if tPA is effective in ischemic stroke, it must be administered as early as possible after the onset of stroke symptoms.

A simplified illustration demonstrates clot breakdown (fibrinolysis), with blue arrows denoting stimulation, and red arrows inhibition. https://en.wikipedia.org/ https://en.wikipedia.org/
6. Human adenosine deaminase (990); Gene therapy for adenosine deaminase; Fabio Candotti,1 Kit L. Shaw,2 Linda Muul, 1 G. Jayashree Jagadeesh1 et al.
https://www.ncbi.nlm.nih.gov;
Ex vivo gene transfer to bone marrow-derived CD34 hematopoietic stem cells (HSCs) of a patient with inherited severe immune deficiency. Autologous HSCs are cultured and infected with a recombinant retroviral or lentiviral vector carrying a functional copy of the defective gene (for example, ADA, gamma-c, or ABCD1). The gene-corrected cells are then injected back into the patient. For some protocols, the patients may receive mild myeloablation prior to infusion of the HSCs.
Adenosine deaminase (ADA) is an enzyme involved in purine metabolism and is essential for lymphocyte development, survival, and function. ADA deficiency is an autosomal-recessive inherited disorder that can result in severe combined immunodeficiency (SCID). Affected infants with ADA-deficient SCID usually are diagnosed by 6 months of age, after presenting with failure to thrive and recurrent opportunistic infections, because of profound pan-lymphopenia and the virtual absence of humoral and cellular immunity. ADA-deficient SCID is almost always fatal by 2 years of age if immunity is not restored.
We conducted a gene therapy trial in 10 patients with adenosine deaminase (ADA)–deficient severe combined immunodeficiency using 2 slightly different retroviral vectors for the transduction of patients' bone marrow CD34+ cells. Four subjects were treated without pretransplantation cytoreduction and remained on ADA enzyme-replacement therapy (ERT) throughout the procedure. Only transient (months), low-level (< 0.01%) gene marking was observed in PBMCs of 2 older subjects (15 and 20 years of age), whereas some gene marking of PBMC has persisted for the past 9 years in 2 younger subjects (4 and 6 years). Six additional subjects were treated using the same gene transfer protocol, but after withdrawal of ERT and administration of low-dose busulfan (65-90 mg/m2). Three of these remain well, off ERT (5, 4, and 3 years postprocedure), with gene marking in PBMC of 1%-10%, and ADA enzyme expression in PBMC near or in the normal range. Two subjects were restarted on ERT because of poor gene marking and immune recovery, and one had a subsequent allogeneic hematopoietic stem cell transplantation. These studies directly demonstrate the importance of providing nonmyeloablative pretransplantation conditioning to achieve therapeutic benefits with gene therapy for ADA-deficient severe combined immunodeficiency.
Human Factor VIII (1040)- Factor VIII (FVIII), an essential blood coagulation protein, is a key component of the fluid phase blood coagulation system. Human factor VIII is a single chain of about 300 kDa consisting of domains described as A1-A2-B-A3-C1- C2. The protein undergoes processing prior to secretion into blood resulting in a heavy chain of 200 kDa (A1-A2-B) and a light chain of 80 kDa (A3-C1-C2) linked by metal ions. The role of factor VIII is to increase the catalytic efficiency of factor IXa in the activation of factor X. Variants of these factors lead frequently also to severe bleeding disorders. http://www.actabp.pl Anna Mazurkiewicz-Pisarek et al
Hepatitis B vaccine SK & F 1986 Hepatitis B vaccine; Hepatitis B vaccine produced cheaply and efficiently using GMO corn. SkepticalRapto According to the World Health Organization, nearly 800,000 individuals die each year worldwide as a result of hepatitis B, an infectious virus that afflicts the liver. A safe and effective vaccine has been available since 1982 and is typically administered via three intramuscular injections over a period of about six months. The vaccine is recommended for babies, children and adults. https://www.geneticliteracyproject.org/

According to the World Health Organization, nearly 800,000 individuals die each year worldwide as a result of hepatitis B, an infectious virus that afflicts the liver. A safe and effective vaccine has been made available since 1982 and is typically administered via three intramuscular injections over a period of about 6 months. The vaccine is recommended for babies, children and adults.
· Engerix B; Hapatitis B vaccination; Recombivax HB: 1 mL (10 mcg) IM at 0, 1, and 6 months. Diabetics who 60 years or older at the discretion of the treating clinician based on increased need for assisted blood glucose monitoring in long-term care facilities, likelihood of acquiring hepatitis B infection, its complications or chronic sequelae, and likelihood of immune response to vaccination.
- Vaccination for older unvaccinated diabetic patients may be done at the physician's discretion MMWR Dec 23, 2011/Vol 60(50);1709-11; http://reference.medscape.com
Digoxin monoclonal:

http://www.medicinenet.com/digoxin/article.htm
Well come 1986 Digoxin antidote; brand name Lanoxin, Lanoxin pediatric. Digoxin is indicated for the treatment of mild to moderate heart failure in adults. Digoxin increases left ventricular ejection fraction and improves heart failure symptoms as evidenced by improved exercise capacity and decreased heart failure-related hospitalizations and emergency care, while having no effect on mortality. Where possible, digoxin should be used in combination with a diuretic and an angiotensin-converting enzyme (ACE) inhibitor.
Digoxin is used to treat heart failure, usually along with other medications. It is also used to treat a certain type of irregular heartbeat (chronic atrial fibrillation); Cardiac glycosides include digoxin, digitoxin, digitalis and ouabain. Of these, only digoxin is in regular use in the UK. Prescribing digoxin is not difficult, providing certain principles are followed. Digoxin acts by inhibiting cell membrane sodium/potassium ATPase which leads to reversal of the usual sodium/calcium exchange. An increased intracellular calcium level results which, in myocardial muscle, has the effect of enhancing the strength of contraction (positive ionotropism). It also affects the electrical physiology of the heart, blocking atrioventricular (AV) conduction and reducing the heart rate by enhancing vagal nerve activity (negative chronotropy). The principal indication is permanent/persistent Atrial fibrillation AF with a fast ventricular rate - although it it is not the preferred first-line medication (especially as digoxin prevents the normal rise in heart rate associated with exertion). A loading dose should be given of 15 micrograms/kg of lean body weight. For a woman with a lean body weight of 50 kg this would work out at a total dose of 15 x 50 = 750 micrograms. Lean body weight is defined as total body mass minus fat mass. There are a number of methods for determining this but where it is clinically significant the simplest method in primary care is the use of skin calipers; Digoxin, sold under the brand name Lanoxin among others, is a medication used to treat various heart conditions. Most frequently it is used for atrial fibrillation, atrial flutter, and heart failure. Digoxin is taken by mouth or by injection into a vein. Digoxin was first isolated in 1930 from the foxglove plant, Digitalis lanata. The most common indications for digoxin are atrial fibrillation and atrial flutter with rapid ventricular response.
Antibody (Digibind)
Orthoclonal OKT3 Cilag 1986 Rejection prophylaxis; Muromonab-CD3 (trade name Orthoclone OKT3, marketed by Janssen-Cilag) is an immunosuppressant drug given to reduce acute rejection in patients with organ transplants. It is a monoclonal antibody targeted at the CD3 receptor,[3] a membrane protein on the surface of T cells. It was the first monoclonal antibody to be approved for clinical use in humans; T cells recognize antigens primarily via the T cell receptor. This receptor needs various co-receptors to function, one of which is CD3. The T cell receptor-CD3 complex transduces the signal for the T cell to proliferate and attack the antigen.
Muromonab-CD3 is a murine (mouse) monoclonal IgG2a antibody which was created using hybridoma technology. It binds to the T cell receptor-CD3-complex (specifically the CD3 epsilon chain) on the surface of circulating T cells, initially leading to activation, but subsequently inducing blockage and apoptosis of the T cells. This protects the transplant against the T cells. After application of muromonab-CD3, normal T cell function is said to be restored within a week. When administered for transplant induction, the drug is administered daily thereafter for up to 7 days.
DIGIBIND, Digoxin Immune Fab (Ovine), is a sterile lyophilized powder of antigen binding fragments (Fab) derived from specific antidigoxin antibodies raised in sheep. Production of antibodies specific for digoxin involves conjugation of digoxin as a hapten to human albumin. Sheep are immunized with this material to produce antibodies specific for the antigenic determinants of the digoxin molecule. The antibody is then papain-digested and digoxin-specific Fab fragments of the antibody are isolated and purified by affinity chromatography. These antibody fragments have a molecular weight of approximately 46,200.
Each vial, which will bind approximately 0.5 mg of digoxin (or digitoxin), contains 38 mg of digoxin-specific Fab fragments derived from sheep plus 75 mg of sorbitol as a stabilizer and 28 mg of sodium chloride. The vial contains no preservatives. DIGIBIND (digoxin immune fab) is administered by intravenous injection after reconstitution with Sterile Water for Injection (4 mL per vial). http://www.rxlist.com.
Somatotropin ( Eli Lilly 1987 ) Growth hormone (Huma trope) deficiency in children; Growth hormone (GH) is a 191 amino-acid single chain polypeptide, which is secreted by the somatotrophs in the anterior pituitary. With the recognition of its multiple and complex effects in the early 1960s, the physiology and regulation of GH has become a major area of research interest in the field of endocrinology. https://www.ncbi.nlm.nih.gov/ Its secretion is regulated by several factors including GHRH, somatostatin, ghrelin and IGF-1. Apart from its primary function of stimulation of GH secretion, GHRH plays an essential role in pituitary somatotroph development and proliferation. Somatostatin on the other hand is the main inhibitor of GH secretion. Along with its receptors, somatostatin has been extensively studied over the last decade as a treatment for acromegaly. Several somatostatin receptors (SSTR) have been identified, of which SSTR2 and SSTR5 exhibit greater inhibition on the secretion of GH by the somatotrophs. Somatostatin receptor ligands to SSTR2 and 5, such as octreotide, lanreotide and pasireotide, are approved treatment modalities for acromegaly in some countries. GH acts both directly through its own receptors and indirectly through the induced production of Insulin-like Growth Factor I (IGF-I). The “IGF-1 axis” holds a significant place in the field of endocrinology, with numerous research been done on its pharmacokinetics and pharmacodynamics, affecting different organ systems in humans. Its physiological effects have been demonstrated not only in tissue growth, but also in glucose / lipid metabolism, coronary disease, diabetes mellitus and vascular aging. The use of recombinant IGF-1 in IGF-1 deficiency and insulin insensitivity and the use of IGF-1 receptor inhibitors in the promotion of cellular apoptosis, especially in the management of malignancies, are two other main areas of research in prospect.
Next Gen, sequencing: http://www.ebi.ac.uk/?
ASPIRIN: William L.smith et al;
Around 400 BC, Hippocrates, the "father of medicine" and the namesake of the Hippocratic oath taken by all physicians, administered willow-leaf tea ; Friedrich Bayer developed aspirin (acetylsalicylic acid) in 1897. Aspirin blocks COX which produces PG. Now more than 80 million tablets of it are being consumed every day in the United States alone, and for many more purposes. Aspirin (Bayer) ,is called as Ecotrin, Ibuprofen (Advil, Motrin), and Naproxen (Aleve). Aspirin is that it irritates the stomach and in some cases cause ulcer and internal bleeding Chemically it is made up of acetylsalicylic acid, a derivative of salicylic acid; notably white willow and meadowsweet (Spirea ulmaria).
Although aspirin was introduced as a pain reliever nearly 100 years ago, how it actually works has remained a mystery until now. It is a non-steroidal anti-inflammatory drugs (NSAIDs). Aspirin like ibuprofen and indomethacin, aspirin emerged in 1899; inhibits an enzyme that produces prostaglandins’ hormone-like messenger molecules that trigger processes in the body, including inflammation.
Prostaglandin H2 synthase, or PGHS. Using X-rays to probe the positions of atoms in tiny crystals of the enzyme, they showed that PGHS has a tunnel running into the middle of it. The raw material must pass through this tunnel to reach the core of the enzyme, where it will be converted into prostaglandin. Aspirin permanently attaches a portion of itself inside the tunnel, where it acts as a gate, blocking prostaglandin's precursor from reaching the "active site" of the enzyme. There are two PGs-PGHS1 and PGHS2. It reduces swelling and is used to treat gout, rheumatoid arthritis, and inflammatory ailments. Many people take low dosages (below 100 milligrams) daily for preventing recurrent stroke or heart attack. Recent studies found it effective in reducing risks for colon and breast cancers. Evidence is accumulating for similar effects in Alzheimer and other diseases.
Aspirin for primary prevention of cardiovascular and all-cause mortality events in diabetes: updated meta-analysis of randomized controlled trials. Nearly 2400 years ago Willow tree extracts were used for headache remedy. It contains Acetylsalicylic acid;
It suppresses the action of the enzyme COX, stops the production of prostaglandin, thus disrupting the pathways to pain, inflammation, elevated temperature, and stomach protection. At the molecular level, aspirin inhibits COX activity by forming a covalent bond with the enzyme. This effectively barricades the COX active site and blocks the synthesis of prostaglandin. COX-2, the therapeutic target of aspirin, is made up of two identical subunits that form a dimer.
Aspirin's acetyl group attaches to serine 530, an amino acid that extends into a narrow, hydrophobic channel within the COX-2 enzyme that leads to its active site. When this amino acid is acetylated, the channel is blocked and arachidonic acid, the precursor to prostaglandin, can no longer reach the COX-2 catalytic site.
Aspirin effectively treats headaches, back and muscle pain, and other general aches and pains. Acts on arthritis; rheumatologic diseases, including osteoarthritis, rheumatoid arthritis, the spondyloarthropathies (inflammatory disorders that involve arthritis with other inflammatory and autoimmune responses), and the arthritis and pleurisy that can accompany lupus. Heart Attack, stroke, fever Aspirin (1930) is derivative of salicylic acid. http://higheredbcs.wiley.com/l ;
How does aspirin accomplish all these effects? The enzyme that metabolizes arachidonic acid, the precursor to the prostaglandin product, is called cyclooxygenase. There are two forms of cyclooxygenase—cyclooxygenase 1 (COX-1), which produces prostaglandins in a normal physiological state; and cyclooxygenase 2 (COX-2), which mediates pain and inflammation in response to tissue damage. Aspirin inhibits both COX-1 and COX-2 irreversibly. While COX-2 is the therapeutic target of aspirin, it is aspirin's interaction with COX-1 in the gastrointestinal tract that causes the drug's unwanted side effects. COX-1 is needed to maintain a thick stomach lining. Because aspirin disables the COX-1 enzyme, regular use of this drug can lead to a thinning of the mucus that protects the stomach from gastric juices. Ulcers, stomach bleeding, and, in some cases, perforation of the stomach can occur. Thus, aspirin has both good and bad effects. It is very effective in alleviating pain through inhibition of the COX-2 pathway. However, when used for long periods and in high doses, aspirin can cause substantial medical problems by stopping the action of COX-1. It Acts on Cancer?
Ecosprin; Ecosprin Tablet is a medicine that is used for the treatment of Blood thinning in heart disease and stroke, Heart attack, Nerve pain, Headache, Migraine, Toothache and other conditions. Ecosprin Tablet contains Aspirin as an active ingredient. Ecosprin Tablet works by reducing prostaglandin thus helping blood thinning and prevents clotting. Detailed information related to Ecosprin Tablet's uses, composition, dosage, side effects and reviews is listed below. Ecosprin Tablet is a medicine that is used for the treatment of Blood thinning in heart disease and stroke, Heart attack, Nerve pain, Headache, Migraine, Toothache and other conditions. It is often called Aspirin delayed release tablets called Ecosprin-75.
Ecosprin tablet contains Aspirin as an active ingredient(Acetyl salicyclic acid). Ecosprin Tablet works by reducing prostaglandin thus helping blood thinning and prevents clotting. Detailed information related to Ecosprin Tablet's uses, composition, dosage, side effects and reviews is listed below. Ecosprin Tablet is a medicine that is used for the treatment of Blood thinning in heart disease and stroke, Heart attack, Nerve pain, Headache, Migraine, Toothache, Sore throat, Joint pain and other conditions. Inactivation of the cyclooxygenase (COX; officially known as prostaglandin-endoperoxide synthase, PTGS). Greek word glukus (γλυκύς), meaning "sweet". The suffix "-ose" denotes a sugar. The name "dextrose" and the 'D-' prefix come from Latin dexter ("right"), referring to the handedness of the molecules. Aspirin (75 Mg) . The treatment of High cholesterol, Heart complications, Blood clots formation in arteries, Stroke, Headache, Migraine and other conditions. Lipikind Plus Capsule contains Aspirin, Atorvastatin, and Clopidogrel as active ingredients. Lipikind Plus Capsule works by blocking the enzyme required for the production of cholesterol in the body; slowing the platelets from sticking to blood vessels; reducing prostaglandin thus helping blood thinning and prevents clotting; http://www.medschat.com/
Saridon: Propyphenazone,
a pyrazolone derivative with anti-inflammatory, analgesic and antipyretic activity, was
introduced in 1951 for the treatment of rheumatic
disorders. Dyscrias; replacement drug for aminophenazone. Saridon was assessed as more efficacious than
ibuprofen, paracetamol, aspirin, and placebo. The currently global base formulation contains 135 mg of propyphenazone,
260 mg of paracetamol and 55 mg of caffeine- fast acting within 15 min. It contains caffeine. The currently global base formulation contains 135 mg of propyphenazone,
260 mg of paracetamol and 55 mg of caffeine- fast acting within 15 min.
Manufacturers of caffeine tablets claim that using caffeine of pharmaceutical quality improves mental alertness- students see at the time of examination (Propyphenazone- non selective COX inhhibitor). The effects of caffeine on learning, memory, performance and coordination are rather related to the methylxanthine action on arousal, vigilance and fatigue. Caffeine exerts obvious effects on anxiety and sleep which vary according to individual sensitivity to the methylxanthine.
Lipikind Plus:
2-Lipikind Plus Capsule is composed of the following active ingredients (salts), Lipikind tablet- Atorvastatin (10 Mg), Fenofibrate , Clopidogrel (75 Mg); Aspirin (75 Mg).
![]()
Lipikind Plus Capsule is composed of the following 2 methoxy-1-naphthadehyde; active ingredients (salts); Lipikind F Tablet works by blocking the enzyme required for the production of cholesterol in the body; increasing the action of enzyme lipoprotein lipase to decrease the level of fats in the blood; active ingredients (salts), Atorvastatin (10 Mg), Clopidogrel (75 Mg), Aspirin (75 Mg). The treatment of High cholesterol, Heart complications, Blood clots formation in arteries, Stroke, Headache, Migraine and other conditions. Lipikind Plus Capsule contains Aspirin, Atorvastatin, and Clopidogrel as active ingredients. Lipikind Plus Capsule works by blocking the enzyme required for the production of cholesterol in the body; slowing the platelets from sticking to blood vessels; reducing prostaglandin thus helping blood thinning and prevents clotting; http://www.medschat.com/ http://www.tabletwise.com/
Pentakind: Pentakind; COMPANY NAME IS MANKIND. This medication contains the active ingredient Pantoprazole, it is a proton pump inhibitor that's used to help reduce the amount of acid produced by the stomach to help prevent or treat conditions such as heartburn, GERD, ulcers and gastritis. Side effects may include nausea, dizziness, flatulence and stomach pain. Pantoprazole, it is a proton pump inhibitor that's used to help reduce the amount of acid produced by the stomach to help prevent or treat conditions such as heartburn, This medication is a proton-pump inhibitor, Nexium-pentakind like; http://www.medschat.com/.
Abilify: Hydrochlorothiazide; Azithromycin; Generic Prilosec ), Generic Norvasc (amlodipine besylate) (omeprazole), Seroquel- antipsychotic, Crestor, a cholesterol-lowering statin drug; Actos, a diabetes drug.
|
Rank |
Drug (Brand Name) |
|
1 |
Abilify |
|
2 |
Nexium |
|
3 |
Humira |
|
4 |
Crestor |
|
5 |
Advair Diskus |
|
6 |
Enbrel |
|
7 |
Remicade |
|
8 |
Cymbalta |
|
9 |
Copaxone |
|
10 |
Neulasta |
|
11 |
Lantus Solostar |
|
12 |
Rituxan |
|
13 |
Spiriva Handihaler |
|
14 |
Januvia |
|
15 |
Atripla |
|
16 |
Lantus |
|
17 |
Avastin |
|
18 |
Lyrica |
|
19 |
Oxycontin |
|
20 |
Epogen |
|
21 |
Celebrex |
|
22 |
Truvada |
|
23 |
Diovan |
|
24 |
Gleevec |
|
25 |
Herceptin |
|
26 |
Lucentis |
|
27 |
Namenda |
|
28 |
Vyvanse |
|
29 |
Zetia |
|
30 |
Levemir |
|
31 |
Symbicort |
|
32 |
Sovaldi |
|
33 |
Novolog Flexpen |
|
34 |
Novolog |
|
35 |
Tecfidera |
|
36 |
Suboxone |
|
37 |
Humalog |
|
38 |
Xarelto |
|
39 |
Seroquel XR |
|
40 |
Viagra |
|
41 |
Alimta |
|
42 |
Victoza 3-Pak |
|
43 |
Avonex |
|
44 |
Nasonex |
|
45 |
Cialis |
|
46 |
Gilenya |
|
47 |
Stelara |
|
48 |
Flovent HFA |
|
49 |
Prezista |
|
50 |
Procrit |
|
51 |
Isentress |
|
52 |
Janumet |
|
53 |
Renvela |
|
54 |
Humalog Kwikpen |
|
55 |
Orencia |
|
56 |
Dexilant |
|
57 |
Vesicare |
|
58 |
Neupogen |
|
59 |
Reyataz |
|
60 |
Lunesta |
|
61 |
Synthroid |
|
62 |
Pradaxa |
|
63 |
Zytiga |
|
64 |
Benicar |
|
65 |
Xolair |
|
66 |
Avonex Pen |
|
67 |
Vytorin |
|
68 |
Invega Sustenna |
|
69 |
Sensipar |
|
70 |
Xgeva |
|
71 |
Prevnar 13 |
|
72 |
Evista |
|
73 |
Aranesp |
|
74 |
Betaseron |
|
75 |
Stribild |
|
76 |
Combivent Respimat |
|
77 |
Xeloda |
|
78 |
Afinitor |
|
79 |
Ventolin HFA |
|
80 |
Synagis |
|
81 |
Zyvox |
|
82 |
Bystolic |
|
83 |
Sandostatin LAR |
|
84 |
Complera |
|
85 |
Benicar HCT |
|
86 |
Treanda |
|
87 |
Gardasil |
|
88 |
Zostavax |
|
89 |
Pristiq |
|
90 |
Erbitux |
|
91 |
Focalin XR |
|
92 |
Tarceva |
|
93 |
Cubicin |
|
94 |
Strattera |
|
95 |
Latuda |
|
96 |
Velcade |
|
97 |
Viread |
|
98 |
Welchol |
|
99 |
Nuvaring |
|
100 |
Sprycel |
Losartan:
Losartan (rINN) /loʊˈsɑːrtən/ is an angiotensin II receptor antagonist drug used mainly to treat high blood pressure (hypertension). It was the first angiotensin II antagonist to be marketed. Losartan potassium is marketed by Merck & Co. Inc. under the trade name Cozaar, and is available in generic form.
The LIFE study demonstrated losartan was significantly superior to atenolol in the primary prevention of adverse cardiovascular events (myocardial infarction or stroke), with a significant reduction in cardiovascular morbidity and mortality for a comparable reduction in blood pressure. Losartan is also available as hydrochlorothiazide/losartan, a combination drug with a low-dose thiazide diuretic to achieve an additive antihypertensive effect. Losartan also causes weight gain. It is also called angiotensin II receptor antagonists. It keeps blood vessels from narrowing, which lowers blood pressure and improves blood flow.
Losartan is used to treat high blood pressure (hypertension). It is also used to lower the risk of stroke in certain people with heart disease. Losartan is used to slow long-term kidney damage in people with type 2 diabetes who also have high blood pressure. It may also delay progression of diabetic nephropathy and is associated with a positive clinical outcome in that regard.
ARB” stands for “angiotensin II receptor blocker (or ACE Inhibitors); ARBs make it impossible for angiotensin to constrict blood vessels by blocking this receptor. This means that ARBs don’t actively cause blood vessels to relax. Rather, they prevent the uptake of a chemical which would cause blood vessels to tense. Relaxed blood vessels help supply adequate blood and oxygen to the heart, which helps it to work efficiently. When arteries are constricted or narrowed, blood is under more pressure because it’s being forced to move through a smaller space than it normally would be.
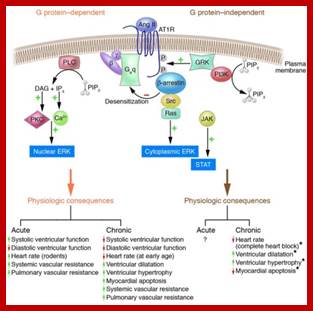
Upon agonist ligand (Ang II) binding to the AT1R, both G protein–dependent and G protein–independent signaling pathways are activated. The AT1R is principally coupled to Gαq, as illustrated, but may also couple to Gαi (not shown). Agonist-induced Gαq activation results in activation of phospholipase C (PLC), which catalyzes breakdown of the membrane lipid phosphatidylinositol-4,5-bisphosphate (PIP2) into inositol triphosphate (IP3) and diacylglycerol (DAG), which respectively act to increase cytosolic Ca2+ concentrations and to activate PKC. Proximally, a series of events results in the inhibition of initiation of Gαq-mediated signaling (desensitization). Agonist-ligand binding induces GRKs, G protein-coupled Receptor Kinase, to catalyze cytoplasmic serine/threonine phosphorylation, which enhances binding of β-arrestin to the AT1R cytoplasmic tail; It sterically inhibits Gαq coupling to the AT1R. The β-arrestin also serves as a scaffold for signaling effectors such as Src, resulting in downstream activation of cytoplasmic ERK. Also, as illustrated, AT1R induces activation of the JAK/STAT pathway. Upon ligand stimulation, the multifunctional enzyme PI3K is recruited to the plasma membrane by interactions with GRK2 (20). In addition to its protein kinase activity, PI3K functions to catalyze the conversion of PIP2 to phoshatidylinositol-3,4,5-triphosphate (PIP3) to promote 7TMR internalization (20). Both G protein–dependent and –independent pathways have distinct physiologic and pathophysiologic effects, as shown. The acute physiologic effects of G protein–independent signaling via AT1R as well as the chronic physiologic effects of G protein–independent signaling on ventricular function and systemic/pulmonary hemodynamics are unknown. The novel physiologic findings of Zhai et al. (3) are each listed and indicated with an asterisk. Peptide hormone Angiotensin II (Ang II) activates NAD(P)H oxidase, via AT1 receptors leading to increased generation of reactive oxygen species (ROS), such as the superoxide anion (O2–).
Potential signaling pathway in Angiotensin II (Ang II) stimulation: Ang II binds to Ang II type I receptors (AT1-R), activates protein kinase C (PKC), which activates NADPH oxidase to induce superoxide (O2−) generation. Mitochondrial KATP channels can be activated by O2− to produce more generation of reactive oxygen species (ROS) to induce activation of mitogen-activated protein kinase (MAPK), which mediates Ang II induced cell proliferation and apoptosis, etc. Angiotensin II receptor blocker (ARB), PKC inhibitor (GFX1092303X), NAD(P)H oxidase inhibitor (Apocynin), Mitochondrial KATP channel inhibitor (5-HD), ERK inhibitor (PD98059), JNK inhibitor (SP600125), p38 inhibitor (SB203580).
Angiotensin II is a hormone; ARBs;
They lower blood pressure through a combination of effects on the heart, Kidney and blood vessels. They decrease the contraction of the heart and dilate the blood vessels. They also help the kidney remove extra salt and water from the body thereby lowering the blood pressure. They prevent calcium from entering the muscle cells of the heart and blood vessels, this relaxing blood vessels and reducing blood pressure. In addition, they are useful in preventing chest pain and slowing dangerously fast heart rhythms. Calcium channel blockers are some of the most commonly prescribed medication for hypertension. Avoid in pregnancy, bradycardia, congestive heart failure.
A study hints that losartan has a beneficial effect on mitochondria by reversing age related dysfunction in maintaining normal blood pressure and cellular energy usage. Competitive angiotensin II receptor type 1 (AT1) ; All of the physiological effects of angiotensin II, including release of aldosterone, are antagonized in the presence of losartan. Reduction in blood pressure occurs independently of the status of the renin-angiotensin system. As a result of losartan dosing, plasma renin activity increases due to removal of the angiotensin II feedback.
It undergoes significant first-pass metabolism to produce the 5-carboxylic acid metabolite, designated as EXP3174. About 14% of an oral dosage is converted to this metabolite, which is long-acting (6 to 8 hr) and a noncompetitive antagonist at the AT1 receptor, contributing to the pharmacological effects of losartan. EXP3174 is 10-40 times more potent in blocking AT1 receptors than losartan. Losartan's bioavailability is about 32%.
Metabolism is primarily by cytochrome P450 isoenzymes CYP2C9 and CYP3A4. Peak plasma concentrations of losartan and EXP3174 occur about one hour and three to four hours, respectively, after an oral dose. Both losartan and EXP3174 are more than 98% bound to plasma proteins. Losartan is excreted in the urine, and in the feces via bile, as unchanged drug and metabolites. About 4% of an oral dose is excreted unchanged in urine, and about 6% is excreted in urine as the active metabolite. The terminal elimination half-life of losartan and EXP3174 are about 1.5 to 2.5 hours and 3 to 9 hours, respectively.
The most common side effects for losartan are upper respiratory infections or stuffy nose, dizziness, and back pain. Your doctor has prescribed Losartan to: treat hypertension (high blood pressure) in adults and in children and adolescents 6-18 years of age; protect the kidney in hypertensive type 2 diabetic patients with laboratory evidence of impaired renal function and proteinuria ≥0.5mg per day (a condition in which urine contains an abnormal amount of protein);reduce the risk of stroke in hypertension with left ventricular hypertrophy (thickening of the heart muscle); treat chronic heart failure when treatment with ACE inhibitors (angiotensin-converting enzyme inhibitors - also used to lower blood pressure) is not considered suitable by your doctor; ACE-angiotensin converting enzyme inhibitors.
Allegra;_Fexofenadine;
Fexidine, Telfast, Fastofen, Tilfur, Vifas, Telfexo, Allerfexo, Flexofen
Name (±)-4-[1 hydroxy-4-[4-(hydroxydiphenylmethyl)-1-piperidinyl]-butyl]-, -dimethyl benzeneacetic acid hydrochloride. Therapeutically, fexofenadine is a selective peripheral H1-blocker (histamine Blockers). Fexofenadine is used for relief from physical symptoms associated with seasonal allergic rhinitis and for treatment of chronic urticaria. Fexofenadine is a selectively peripheral H1-blocker. Blockage prevents the activation of the H1 receptors by histamine, preventing the symptoms associated with allergies from occurring.
Fexofenadine hydrochloride is 60% to 70% bound to plasma protein, primarily albumin and 1-acid glycoprotein. The half-life is 14.4 hours for 60 mg. The drug works by competitively inhibiting the H-1 receptor site and preventing histamine-1 from attaching the receptor sites to cause allergy symptoms. Histamine-1 is released by mast cells in the presence of certain stimulus that the body is allergic to. The drug does not prevent the release of histamine-1 by the mast cells but rather competes with the histamine-1 substrates for positions at the receptor sites. Claritin (loratadine) and Zyrtec (cetirizine) are alternatives to Allegra(Fexofenadine).
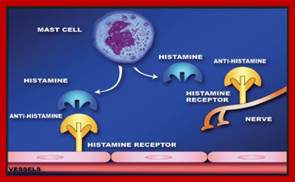
https://en.wikibooks.org/ http://www.medicinenet.com/fexofenadine-oral/article.htm;
Fexofenadine-2D structure; https://pubchem.ncbi.nlm.nih.gov
When your body is exposed to allergens, it reacts by releasing histamines that attack cells in the body, causing them to swell up and leak fluid. This results in the common allergy symptoms of watery eyes, congestion, swelling of the nasal passages, itching, sneezing and runny nose. The most common over-the-counter antihistamine medications, known as first-generation antihistamines, work by blocking histamines. However, they also cross the blood-brain barrier, interacting with a number of unintended receptor sites. Used for headache, dizziness, diarrhea, vomiting, pain in the arms, legs, or back, pain during menstrual period cough.
Vitamins:
Very important biochemical required for all animals and plants for their growth and reproduction. There are 15-20 vitamins. Animals cannot synthesize tem and they have to obtain from other sources. Plants synthesize most of the vitamins and they are self-sufficient and animals are dependent on plants for their vitamin sources. Vitamins act as coenzymes, signaling molecules, antioxidants and some act as hormones and few other important functions. Vitamins deficiency is noted morphologically in some and many other in physiological functions and they are functionally identifiable. Basically the vitamins are classifies based their solubility in water and fat.
Water soluble- Thiamin, Riboflavin, Niaicin, Vitamin B12, Folate, Vitamin B12, Pantothenic acid, Biotin, and Vitamin C.
Fat soluble- Vitamin A, Vitamin d, Vitamin E, and Vitamin K. Vitamins act as coenzymes. http://www.duhs.edu.pk; https://en.wikibooks.org.
Water soluble Vitamins: As they water soluble they also get excreted from the body easily. Excess drinking water can cause some vitamin deficiency; common vitamin deficiency is Vit B forms
Thiamin: Its deficiency causes beri-beri- dry (paralytic) and wet (oedematous) forms; more rice and starch product consuming causes- Beri-Beri. Deficiency can cause the neurologic reflections of Wernicke-Korsakoff syndrome are frequently associated with chronic alcoholism with limited food consumption. Toxicity is not a problem with thiamin because renal clearance of levels conceivably ingested is rapid.
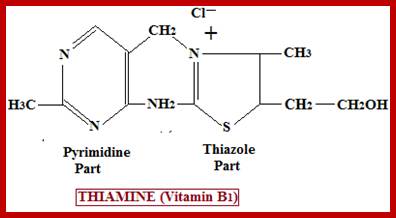
http://www.studyandexam.com
It functions as co-enzyme of thiamine pyrophosphate in carbohydrate metabolism. Deficiency causes loss in carbohydrate metabolism. Best dietary sources are whole grains, legumes, beef, liver, nuts, and yeast while eggs, fish and vegetables contain it to a lesser extent.
Vitamin C: Vitamin C, is called Ascorbic acid, is a powerful antioxidant with a vast number of health benefits, including its ability to act as an effective antihistamine. Rather than blocking histamine from binding to receptor sites, as pharmaceuticals D. Vitamin C is synthesized in the liver in some mammals. Deficiency causes scurvy.
https://faq.soylent.com
Vitamin C benefits for allergy sufferers occur because it prevents histamine from forming in the first place. Vitamin C also reduces inflammation by helping to better balance immune responses. Plan to take about 1 gram three to five times per day to relieve symptoms. If symptoms persist … you need more. Vitamin C is an electron donor (reducing agent or antioxidant), and probably all of its biochemical and molecular functions can be accounted for by this function. The potentially protective role of vitamin C as an antioxidant is discussed in the antioxidants chapter of this report. Ascorbate is found in many fruits and vegetables (75). Citrus fruits and juices are particularly rich sources of vitamin C but other fruits including cantaloupe, honeydew melon, cherries, kiwi fruits, mangoes, papaya, strawberries, tangelo, watermelon, and tomatoes also contain variable amounts of vitamin C. Vegetables such as cabbage, broccoli, Brussels sprouts, bean sprouts, cauliflower, kale, mustard greens, red and green peppers, peas, tomatoes, and potatoes may be more important sources of vitamin C than fruits. This is particularly true because the vegetable supply often extends for longer periods during the year than does the fruit supply.
For more information about the best way to take vitamin C – read about the ‘multi-C protocol’ by Thomas E. Levy, MD, JD.
Riboflavin: It is Vitamin B2. It prevents migraines’. It is required for good body respiration. Riboflavin supplements have been used as part of the phototherapy treatment of neonatal jaundice.
Collagen cross-linking is a non-surgical treatment intended to slow progression of corneal ectasia by strengthening corneal tissue. The standard protocol calls for application directly to the eye of a 0.1% riboflavin solution for 30 minutes followed by 30 minutes of ultraviolet-A irradiation with wavelength of 370 nm and power of 3 mW/cm.
Riboflavin functions as a coenzyme in the form of Flavin mononucleotide FMN and Flavin adenine dinucleotide FAD. They ae involved in electron transport chain, they are involved in fatty acid oxidation and many such components. They are very important coenzymes. These vitamins are found in milk, cheese, eggs, leaf vegetables, liver, kidneys, legumes, mushrooms, and almonds; The stomatitis symptoms are similar to those seen in pellagra, which is caused by niacin (B3) deficiency. Therefore, riboflavin deficiency is sometimes called "pellagra sine pellagra" (pellagra without pellagra), because it causes stomatitis but not widespread peripheral skin lesions characteristic of niacin deficiency
Vitamin B12. It is an organometallic compound, mde up of Methyl group, deoxyadenosine, cyanide group too. Often it is called Cynocobalamin.
Anerobic bacteria can convert Co, CO2 and lignin by degradation into Acetyl Co A. It has a uniques corrin ring. All re cobalamins contain in their molecules a portion called corrin ring which to a large extent resembles the tetrapyrrole ring structure of porphyrins. It has CN, OH, H2O and NO2 groups. The ring structure looks more like chlorophyll ion rings. There are three coenzymes of this type which differ from each other in respect of benzimidazole portion of the vitamin B12 molecule. One of these has 5,6,dimethyl benzimidazole, the second has benzimidazole and the third has adenyl group; they are respectively called DBC, BC and AC. In human liver DBC is the most predominant coenzyme B12. Methyle group: A coenzyme B12 containing a methyl group attached to the Co atom has also been discovered.
Folate; Folic acid is one of the B-complex vitamins required in order to produce red blood cells. Folic acid is a manufactured form of folate; folate can be found naturally in certain foods, such as leafy green vegetables, nuts, beans, and grains. Some cereals contain 100% of the daily value of folic acid a woman should take per day. If there is an insufficient amount of this vitamin, it can cause anemia. Tongue get boils.
![]()
https://en.wikibooks.org
Because the body does not make much folic acid, it is useful to take a vitamin pill form to ensure that you get the recommended daily value. folate can be found naturally in certain foods, such as leafy green vegetables, nuts, beans, and grains. Some cereals contain 100% of the daily value of folic acid a woman should take per day. Folates decrease eart diseases. Since many cancer cells tolerate folic acid and overexpress the folic acid receptor, this had led to the creation of anti-cancer drugs that target the folic acid receptor. Folates decrease the risk of colorectal cancer. https://en.wikibooks.org
Pantothenic acid- It is also known as Vitamin B5. It is the precursor for the synthesis of Co Enzyme A. It acts as an acyl carrier.
http://www.studyandexam.com;study skills
Evidence from limited intervention studies suggests that pantothenic acid and/or pantothenol (alcohol analog) might improve the healing process of skin wounds. Yet, additional larger studies are warranted. It is now claried that it reduces blood cholesterol. Milk products, eggs, avocados, legumes, mushrooms, and sweet potatoes are rich in pantothenic acid. It is found in the form of Coenzyme A, which has many roles in biochemical reactions. It has been found it has important role in wound healing.
![]()
Biotin: It was once called Vitamin H or Co enzyme R. It is composed of a ureido ring fused with a tetrahydrothiophene ring. A valeric acid substituent is attached to one of the carbon atoms of the tetrahydrothiophene ring. Biotin is a coenzyme for carboxylase enzymes, involved in the synthesis of fatty acids, isoleucine, and valine, and in gluconeogenesis. https://en.wikipedia.org.

Deficiency can cause hair thinning, skin rash. Biotin acts as a coenzyme for carboxylase enzymes, involved in the synthesis of fatty acids, isoleucine, and valine, and in gluconeogenesis. It is involved in fatty acid synthesis and metabolism and also of aminoacyls. It is helpful in maintaining steady blood flow. Surprisingly intestinal bacteria synthesize Biotin. It acts as a cofactors with Acetyl CoA carboxylase alpha and beta, pyruvate carboxylase, Methyl crotonyl- CoA carboxylae, Pyruvate carboxylase. It is important I fatty acid synthesis, amino acid catabolism and gluconeogenesis. Best source of Biotins are soybean, sunflower seeds, green peas, lentils, peanuts etc. Eggs white (contains Avidin) which blocks absorption of biotin.
Fat soluble Vitamins:
Vitamin A: Vitamin A (retinol) is an essential nutrient needed in small amounts by humans for the normal functioning of the visual system; growth and development; and maintenance of epithelial cellular integrity, immune function, and reproduction. These dietary needs for vitamin A are normally provided for as preformed retinol (mainly as retinyl ester) and pro-vitamin A carotenoids. Tissues extract most lipids and some carotenoids from circulating chylomicrons, but most retinyl esters are stripped from the chylomicron remnant, hydrolysed, and taken up primarily by parenchymal liver cells. If not immediately needed, retinol is re-esterified and retained in the fat-storing cells of the liver (variously called adipocytes, stellate cells, or Ito cells). The liver parenchymal cells also take in substantial amounts of carotenoids. Whereas most of the body's vitamin A reserve remains in the liver, carotenoids are also deposited elsewhere in fatty tissues throughout the body (1). Usually, turnover of carotenoids in tissues is relatively slow, but in times of low dietary carotenoid intake, stored carotenoids are mobilised. A recent study in one subject using stable isotopes suggests that retinol can be derived not only from conversion of dietary pro-vitamin carotenoids in enterocytes - the major site of bioconversion - but also from hepatic conversion of circulating pro-vitamin carotenoids (5). The quantitative contribution to vitamin A requirements of carotenoid converted to retinoids beyond the enterocyte is unknown. http://www.fao.org
Biochemical mechanisms for vitamin A functions:
Vitamin A functions at two levels in the body. The first is in the visual cycle in the retina of the eye; the second is in all body tissues systemically to maintain growth and the soundness of cells. In the visual system, carrier-bound retinol is transported to ocular tissue and to the retina by intracellular binding and transport proteins. Rhodopsin, the visual pigment critical to dim-light vision, is formed in rod cells after conversion of all-trans retinol to retinaldehyde, isomerization to the 11-cis-form, and binding to opsin. Alteration of rhodopsin through a cascade of photochemical reactions results in ability to see objects in dim light (13). The speed at which rhodopsin is regenerated relates to the availability of retinol. Night blindness is usually an indicator of inadequate available retinol, but it can also be due to a deficit of other nutrients, which are critical to the regeneration of rhodopsin, such as protein and zinc, and to some inherited diseases, such as retinitis pigmentosa.
Some important facts on vitamin-A
- Approximately 50-85% of total body retinol is stored in the liver and more than 90% of this as retinyl esters (RE)157
- Retinal plays an important part in vision – it is a structural component of rhodopsin or visual purple, which is the light sensitive pigment within rod and cone cells of the retina.
- Retinoic acid (RA) affects the transcription of many genes.
- Without enough protein and amino acids, transport proteins such as RBP or TTR (Transthyretin) cannot be formed and thus retinol cannot be transported. So Protein Energy Malnutrition is of concern in developed and developing countries as a cause of Vitamin-A deficiency. Up to 65% of patients in hospital could be malnourished220,222 and it has been estimated that around 33% of people over 65 years of age in the US and UK eat a diet that is deficient in both protein and calories221. This places such an individual at great risk for sepsis (infection) and increased morbidity and mortality. The British Association of Parenteral and Enteral Nutrition (BAPEN), estimates 10,000 lives could be saved if the possibility of malnutrition was actively assessed at the time of admission and then corrected. A significant protein energy malnutrition will worsen a pre-existing vitamin-A deficiency putting the patient at high risk for an infective complication. All of this is worsened because any acute illness or surgical intervention will increase the need for nutritional resources greatly in an individual already depleted of nutritional resources. The triage theory of nutritional resource deployment in times of scarcity, will be working overtime - the end result, a greatly weakened individual. TTR is a retinol transport protein given the initials TTR because it transports thyroxine and retinol. Retinoic Acid (RA) is the most potent metabolite of Vitamin-A.
Esterified retinol is the form found in greatest concentrations in most tissues. "Vitamin A deficiency is a nutritionally-acquired immunodeficiency disorder characterized by widespread pathological alterations of mucosal epithelia of the respiratory, gastrointestinal, and genito-urinary tracts, impaired antibody responses to protein antigens, altered cell-mediated immunity, and alterations in T-cell subsets; http://www.nutridesk.com.au
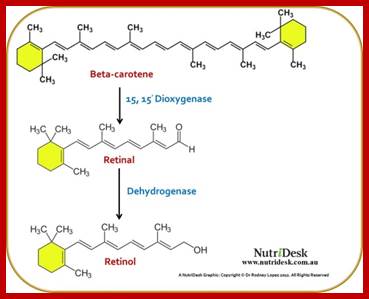
http://www.nutridesk.com.au
Vitamin D, (Calcitrol); Vitamin D exists in two forms, vitamin D2 and vitamin D3, which differ in the structure of their side chains. These are called ergocalciferol and cholecalciferol respectively. Both forms are equivalent as to their biological activity and equivalent in dosage. Both are metabolized by conversion to the 25-hydroxy form and then to the 1,25-dihydroxy metabolite in the kidney, which is the bioactive form. This has a structure which is similar to other steroid hormones produced in the body. Vitamin D is a derivative of 7-dehydrocholesterol, also called ergosterol. This conversion is mediated by the action of ultraviolet radiation the parent compound, which is formed in the Malpighian layer of skin during a relatively minor route of cholesterol synthesis. If we expose to bright sunlight, we produce Vit D naturally. It plays an important role in regulating inaugumnetin the levls of Calcium and Phosphorous
Cholecalciferol is then carried to the liver, where a mitochondrial hydroxylase enzyme introduces a hydroxyl group at the 25 position. This reaction requires both energy in the form of NADPH and oxygen. The product, called 25-hydroxy cholecalciferol, is the inactive
Storage form of cholecalciferol, and is stored in the liver. 1,25-dihydroxy cholecalciferol, also called calcitriol, is carried in the bloodstream to the intestinal mucosa. There it stimulates the absorption of calcium and phosphate, the mineral ions which are of prime importance in the building up of bone and other supportive tissue. It also promotes bone growth and remodeling by osteoblasts and osteoclasts.
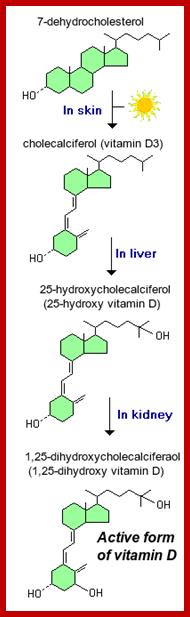
- Within the liver, cholecalciferal is hydroxylated to 25-hydroxycholecalciferol by the enzyme 25-hydroxylase.
- Within the kidney, 25-hydroxycholecalciferol serves as a substrate for 1-alpha-hydroxylase, yielding 1,25-dihydroxycholecalciferol, the biologically active form.
Active form of Vitamin D binds to it secretor at intercellular level, his leads to activation of specific gene transcription. Vitamin D is well known as a hormone involved in mineral metabolism and bone growth. Its most dramatic effect is to facilitate intestinal absorption of calcium, although it also stimulates absorption of phosphate and magnesium ions. In the absence of vitamin D, dietary calcium is not absorbed at all efficiently. Vitamin D stimulates the expression of a number of proteins involved in transporting calcium from the lumen of the intestine, across the epithelial cells and into blood. The best-studied of these calcium transporters is calbindin, an intracellular protein that ferries calcium across the intestinal epithelial cell. Vitamin D, a seco-steroid, can either be made in the skin from a cholesterol-like precursor (7-dehydrocholesterol) by exposure to sunlight or can be provided pre-formed in the diet . http://www.fao.org
Within the liver, cholecalciferal is hydroxylated to 25-hydroxycholecalciferol by the enzyme 25-hydroxylase.
Within the kidney, 25-hydroxycholecalciferol serves as a substrate for 1-alpha-hydroxylase, yielding 1,25-dihydroxycholecalciferol, the biologically active form. It is advised to consumevit,D with calcium one tablet daily
Vitamin D deficiency: The classical manifestations of vitamin D deficiency is rickets, which is seen in children and results in bony deformaties including bowed long bones. Deficiency in adults leads to the disease osteomalacia. Both rickets and osteomalacia reflect impaired mineralization of newly synthesized bone matrix, and usually result from a combination of inadequate exposure to sunlight and decreased dietary intake of vitamin D. This can also lead to Rickets seen in children resulting in bone deformation , bowled bones. Both rickets and osteomalacia reflect impaired mineralization of newly synthesized bone matrix, and usually result from a combination of inadequate exposure to sunlight and decreased dietary intake of vitamin D. it can aslo cause liver and kidney diseases. It is advisable for people t expose to sunlight frequently ans sufficiently. http://www.vivo.colostate.edu/hbooks
Vitamin E, Vitamin E is a fat-soluble vitamin. It is comprised of a family of hydrocarbon compounds characterized by a chromanol ring with a phytol side chain referred to as tocopherols and tocotrienols. Tocopherols possess a saturated phytol side chain whereas the side chain of tocotrienols have three unsaturated residues. Isomers of these compounds are distinguished by the number and arrangement of methyl substituents attached to the chromanol ring. The predominant isomer found in the body is alpha-tocopherol, which has three methyl groups in addition to the hydroxyl group attached to the benzene ring. The diet of animals is comprised of different proportions of tocopherol isomers and specific alpha-tocopherol-binding proteins are responsible for retention of this isomer in the cells and tissues of the body. Because of the lipophilic properties of the vitamin it partitions into lipid storage organelles and cell membranes. It is, therefore, widely distributed in throughout the body. Subcellular distribution of alpha-tocopherol is not uniform with lysosomes being particularly enriched in the vitamin compared to other subcellular membranes. Vitamin E is believed to be involved in a variety of physiological and biochemical functions. The molecular mechanism of these functions is believed to be mediated by either the antioxidant action of the vitamin or by its action as a membrane stabiliser. Alpha-Tocopherol is an efficient scavenger of lipid peroxyl radicals and, hence, it is able to break peroxyl chain propagation reactions.
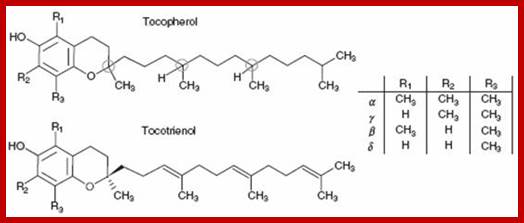
http://www.medandlife.ro/
The unpaired electron of the tocopheroxyl radical thus formed tends to be delocalised rendering the radical more stable. The radical form may be converted back to alpha-tocopherol in redox cycle reactions involving coenzyme Q. The regeneration of alpha-tocopherol from its tocopheroxyloxyl radical greatly enhances the turnover efficiency of alpha-tocopherol in its role as a lipid antioxidant. Vitamin E forms complexes with the lysophospholipids and free fatty acids liberated by the action of membrane lipid hydrolysis. Both these products form 1:1 stoichiometric complexes with vitamin E and as a consequence the overall balance of hydrophobic:hydrophillic affinity within the membrane is restored. In this way, vitamin E is thought to negate the detergent-like properties of the hydrolytic products that would otherwise disrupt membrane stability. The location and arrangement of vitamin E in biological membranes is presently unknown. There is, however, a considerable body of information available from studies of model membrane systems consisting of phospholipids dispersed in aqueous systems. From such studies using a variety of biophysical methods, it has been shown that alpha-tocopherol intercalates into phospholipid bilayers with the long axis of the molecule oriented parallel to the lipid hydrocarbon chains. The molecule is able to rotate about its long axis and diffuse laterally within fluid lipid bilayers. The vitamin does not distribute randomly throughout phospholipid bilayers but forms complexes of defined stoichiometry which coexist with bilayers of pure phospholipid. alpha-Tocopherol preferentially forms complexes with phosphatidylethanolamines rather than phosphatidylcholines, and such complexes more readily form nonlamellar structures. The fact that alpha-tocopherol does not distribute randomly throughout bilayers of phospholipid and tends to form nonbilayer complexes with phosphatidylethanolamines would be expected to reduce the efficiency of the vitamin in its action as a lipid antioxidant and to destabilise rather than stabilise membranes. The apparent disparity between putative functions of vitamin E in biological membranes and the behaviour in model membranes will need to be reconciled. https://www.ncbi.nlm.nih.gov
Vitamin K. This chapter discusses the vitamin K–dependent formation of bone γ-carboxyglutamic acid (Gla) protein (BGP, osteocalcin) and its function. The bone Gla protein (BGP, osteocalcin) is the best characterized noncollagenous bone protein. Characteristic chemical features are small size—typically 49 or 50 residues—and existence within the molecule of three residues of the vitamin K–dependent amino acid, Gla. BGP is among the most abundant noncollagenous bone proteins and appears to be a universal constituent of the skeleton and tooth dentin of all vertebrates. As its synthesis is regulated by 1,25-dihydroxyvitamin D3, it is likely that BGP plays a role in the action of this hormone on bone. BGP has been detected in the calcified tissues of all vertebrates examined to date. The molecular weights of calf and chicken BGP have been determined by sedimentation equilibrium centrifugation and are 5800 and 6500, respectively. Both molecular weight values agree well with those computed from the corresponding covalent structures. In contrast, the apparent molecular weight of BGP determined by gel filtration and by SDS-gel electrophoresis is about 12,000. http://www.sciencedirect.com
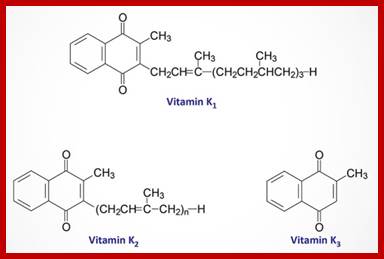
http://themedicalbiochemistrypage.org
Vitamin K is a cofactor required for the formation of γ-carboxyglutamate (Gla) residues in proteins. Osteoblasts produce at least three different Gla-containing proteins: osteocalcin, matrix Gla-protein, and protein S. After cellular secretion of these proteins, the main part of each remains bound to the hydroxyapatite matrix in bone, but their function remains unclear. Part of the newly synthesized osteocalcin is also set free into the bloodstream, where it may be used as a diagnostic marker for bone formation. Several studies have demonstrated that a poor vitamin K status is associated with an increased risk of osteoporotic bone fractures. Whether vitamin K supplementation will reduce the rate of bone loss in postmenopausal women remains a matter of debate.
Antibiotics;
Most of antibiotics inhibit DNA synthesis, RNA synthesis, cell wall synthesis, or protein synthesis.
Amoxicillin: As a derivative of ampicillin, amoxicillin is a member of the same family as penicillins and, like them, is a β-lactam antibiotic.[28] It acts by inhibiting the synthesis of bacterial cell walls. It inhibits cross-linkage between the linear peptidoglycan polymer chains that make up a major component of the cell wall of Gram-positive and a minor component of Gram-negative bacteria. Gram negative bacteria are not generally susceptible to Beta-lactam antibiotics.
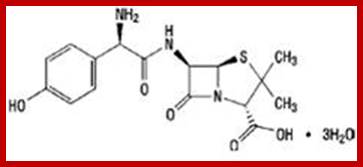
http://www.antibiotics-info.org/
Derivatives of 6-aminopenicillanic acid (6-APA) ; They are derived from Penicillium fungi. Amoxicillin is used in the treatment of a number of infections, including acute otitis media, streptococcal pharyngitis, pneumonia, skin infections, urinary tract infections, Salmonella infections, Lyme disease, and chlamydia infections; https://en.wikipedia.org/ . Amoxillin works by blocking bacterial cell wall synthesis.
Codeine, Codeine is an opiate used to treat pain, as a cough medicine, and for diarrhea. It is typically used to treat mild to moderate degrees of pain. Greater benefit may occur when combined with paracetamol (acetaminophen) or a nonsteroidal anti-inflammatory drug (NSAID) such as aspirin or ibuprofen- https://en.wikipedia.org.
https://www.drugbank.ca/
Codeine is marketed as both a single-ingredient drug and in combination preparations with paracetamol (as co-codamol: e.g., brands Paracod, Panadeine, and the Tylenol-with-codeine series, including Tylenol 3 and 1,2,4); with aspirin (as co-codaprin); or with ibuprofen (as Nurofen Plus). These combinations provide greater pain relief than either agent alone (drug synergy).
Doxycycline, Doxycycline is an antibiotic that is used in the treatment of a number of types of infections caused by bacteria and protozoa. It is useful for bacterial pneumonia, acne, chlamydia infections, early Lyme disease, cholera and syphilis. https://en.wikipedia.org; In addition to the general indications for all members of the tetracycline antibiotics group, belong to tetracyclines’, diahhrea, vision loss. Doxycycline is a synthetic tetracycline derivative with similar antimicrobial activity.
https://pubchem.ncbi.nlm.nih.gov;http://www.healthline.com/
Formation of the 30S initiation complex (made up of mRNA, the 30S ribosomal subunit, and formyl-methionyl-transfer RNA); formation of the 70S ribosome by the 30S initiation complex and the 50S ribosome, elongation process of assembling amino acids into a polypeptide.
Loratadine; Loratadine is an antihistamine that reduces the effects of natural chemical histamine in the body. Histamine can produce symptoms of sneezing, itching, watery eyes, and runny nose. https://www.drugs.com/. A large study links a significantly increased risk for developing dementia, including Alzheimer's disease, to taking commonly used medications with anticholinergic effects at higher doses or for a longer time. Many older people take these medications, which include nonprescription diphenhydramine (Benadryl). JAMA Internal Medicine published the report, called "Cumulative Use of Strong Anticholinergic Medications and Incident Dementia.
https://toxnet.nlm.nih.gov
Loratidine is a piperidine histamine H1-receptor antagonist with anti-allergic properties and without sedative effects. Loratadine blocks the H1 histamine receptor and prevents the symptoms that are caused by histamine activity on capillaries, bronchial smooth muscle, and gastrointestinal smooth muscle, including vasodilatation, increased capillary permeability, bronchoconstriction, and spasmodic contraction of gastrointestinal smooth muscle. Loratadine does not cross the blood-brain barrier and does not cause central nervous system effects. https://www.sciencedaily.com; https://pubchem.ncbi.nlm.nih.gov; https://www.drugbank.ca
Cefalexin, Cefalexin is a beta-lactam antibiotic within the class of first-generation cephalosporins. Cephalexin Anhydrous is the anhydrous form of cephalexin, a semisynthetic first-generation cephalosporin with antibacterial activity. Cephalexin binds to and inactivates penicillin-binding proteins (PBPs) located on the inner membrane of the bacterial cell wall. PBPs are enzymes involved in the terminal stages of assembling the bacterial cell wall and in reshaping the cell wall during growth and division. Inactivation of PBPs interferes with the cross-linkage of peptidoglycan chains necessary for bacterial cell wall strength and rigidity. This results in the weakening of the bacterial cell wall and causes cell lysis. It kills gram-positive and some gram-negative bacteria by disrupting the growth of the bacterial cell wall. As cefalexin closely resembles d-alanyl-d-alanine, an amino acid ending on the peptidoglycan layer of the cell wall, it is able to irreversibly bind to the active site of PBP, which is essential for the synthesis of the cell wall. It is most active against gram-positive cocci, and has moderate activity against some gram-negative bacilli. However, some bacterial cells have the enzyme β-lactamase, which hydrolyzes the beta-lactam ring, rendering the drug inactive. This contributes to antibacterial resistance towards cephalexin.
https://pubchem.ncbi.nlm.nih.go
It kills gram-positive and some gram-negative bacteria by disrupting the growth of the bacterial cell wall. Cefalexin is a beta-lactam antibiotic within the class of first-generation cephalosporins.
Neomycin, Neomycin is a bactericidal aminoglycoside antibiotic that binds to the 30S ribosome of susceptible organisms. Binding interferes with mRNA binding and acceptor tRNA sites and results in the production of non-functional or toxic peptides. http://www.health24.com/ Neomycin is part of the aminoglycoside group of bacteria. It kills bacteria as a result of abnormal protein production in the bacterial cell - it cannot produce any of its proteins correctly - and also as a result of damage to the bacterial cell membrane. The question is , how bacteria survives when it produces such proteins.
http://pubs.rsc.org/
Neomycin that is produced by Streptomyces fradiae, on hydrolysis it yields neamine and neobiosamine B. (From Merck Index, 11th ed). Neomycin is a bactericidal aminoglycoside antibiotic that binds to the 30S ribosome of susceptible organisms. Binding interferes with mRNA binding and acceptor tRNA sites and results in the production of non-functional or toxic peptides.
Streptomycin, It is an aminoglycoside antibiotic. RNAs have diverse structures that include bulges and internal loops able to form tertiary contacts or serve as ligand binding sites. The recent increase in structural and functional information related to RNAs has put them in the limelight as a drug target for small molecule therapy. In addition, the recognition of the marked difference between prokaryotic and eukaryotic rRNA has led to the development of antibiotics that specifically target bacterial rRNA, reduce protein translation and thereby inhibit bacterial growth. To facilitate the development of new antibiotics targeting RNA, we here review the literature concerning such antibiotics, mRNA, riboswitch and tRNA and the key methodologies used for their screening. RNAs have diverse structures that include bulges and internal loops able to form tertiary contacts or serve as ligand binding sites.
https://pubchem.ncbi.nlm.nih.gov
The recent increase in structural and functional information related to RNAs has put them in the limelight as a drug target for small molecule therapy. In addition, the recognition of the marked difference between prokaryotic and eukaryotic rRNA has led to the development of antibiotics that specifically target bacterial rRNA, reduce protein translation and thereby inhibit bacterial growth. To facilitate the development of new antibiotics targeting RNA, we here review the literature concerning such antibiotics, mRNA, riboswitch and tRNA and the key methodologies used for their screening. http://www.sciencedirect.com/ ribonucleic acid (RNA) can fold into numberless tertiary structures that reflect its diverse functions. Thus it serves as the genetic material in some viruses, as the mediator of genetic information from DNA to protein, as the structural component in many ribonucleoproteins (RNPs) and, in some cases, as a catalyst2. RNA is usually associated with RNA-binding proteins (RBPs) which serve to either protect, stabilize or transport it and regulate its interaction with other molecules3 and 4. RNA plays many crucial roles in protein synthesis, transcriptional regulation and retroviral replication that make it a prime target for drug action.
Streptomycin disturbs several steps of protein synthesis leading to translational errors and slowdown of translocation. It binds firmly to a single site on 16S rRNA without binding to the ribonucleoprotein39 as supported by foot printing and mutation studies. Foot-printing studies showed that streptomycin protects specific residues of 16S rRNA within the 30S subunit40 and can be linked to specific portions of 16S rRNA41. Moreover, a mutation in Euglena chloroplast 16S rRNA resulted in streptomycin resistance 42 and mutations in different regions of Escherichia coli 16S rRNA changed the ribosomal response to streptomycin . Streptomycin also interacts with ribosomal proteins in the 30S subunit and mutations in S4, S5 and S12 ribosomal proteins are shown to influence its bindin,
Rifampicin (Rif); Rifampicin inhibits bacterial DNA-dependent RNA synthesis by inhibiting bacterial DNA-dependent RNA polymerase, Taq polymerase; https://en.wikipedia.org
https://pubchem.ncbi.nlm.nih.gov
Rifampicin (Rif) is one of the most potent and broad spectrum antibiotics against bacterial pathogens and is a key component of anti-tuberculosis therapy, stemming from its inhibition of the bacterial RNA polymerase (RNAP). We determined the crystal structure of Thermus aquaticus core RNAP complexed with Rif. The inhibitor binds in a pocket of the RNAP β subunit deep within the DNA/RNA channel, but more than 12 Å away from the active site.
The structure, combined with biochemical results, explains the effects of Rif on RNAP function and indicates that the inhibitor acts by directly blocking the path of the elongating RNA when the transcript becomes 2 to 3 nt in length. Acts on bacteria Gram positive type, RIF activates the human glucocorticoid receptor and acts as a immunodepressant; it acts and inhibits transcription by binding to RNA pol. http://what-when-how.com/molecular-biolog. http://www.sciencedirect.com/
Tetracyclines: Tetracyclines are a broad spectrum of antibiotics. Inhibit protein synthesis by binding to 30S ribosomal subunit and prevent the binding of a.a-tRNA, thus inhibit their steps in translation.
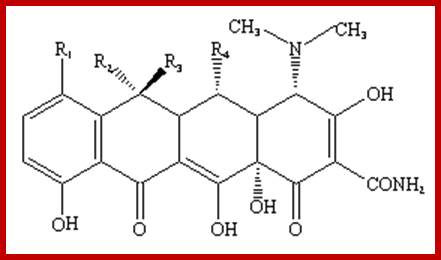
http://textbookofbacteriology.net
Tetrayclines and related doxycyclines, minocycles all block translation of mRNA, for they bind to 30S ribosomes at A site, as a result new aminoacyl tRNAs fail to bind to A site thus translation is inhibited thus cause the death of bacterial cells.
Chloramphenicol: It is an anti microbial and anticancer drug. Chloramphenicol resistance may be carried on a plasmid that also codes for resistance to other drugs. One example is the ACCoT plasmid , Crystal structure data and biochemical data suggest that rifampicin binds to the pocket of the RNA polymerase β subunit within the DNA/RNA channel, but away from the active site. (A=ampicillin, C=chloramphenicol, Co=co-trimoxazole, T=tetracycline), which mediates multiple-drug resistance in typhoid (also called R factors). Chloramphenicol is a bacteriostatic by inhibiting protein synthesis. It prevents protein chain elongation by inhibiting the peptidyl transferase activity of the bacterial ribosome. It specifically binds to A2451 and A2452 residues[36] in the 23S rRNA of the 50S ribosomal subunit, preventing peptide bond formation.[37] While chloramphenicol and the macrolide class of antibiotics both interact with ribosomes, chloramphenicol is not a macrolide. It directly interferes with substrate binding, whereas macrolides sterically block the progression of the growing peptide. https://en.wikipedia.org/ Crystal structure data and biochemical data suggest that rifampicin binds to the pocket of the RNA polymerase β subunit within the DNA/RNA channel, but away from the active site; By this "steric-occlusion" mechanism, rifampicin blocks synthesis of the second or third phosphodiester bond between the nucleotides in the RNA backbone, preventing elongation of the 5' end of the RNA transcript past more than 2 or 3 nucleotides. https://en.wikipedia.org/
Erythromycin, Erythromycin is a macrolide antibiotic produced by Saccharopolyspora erythraea (formerly Streptomyces erythraeus). It inhibits bacterial protein synthesis by binding to bacterial 50S ribosomal subunits; binding inhibits peptidyl transferase activity and interferes with translocation of amino acids during translation and assembly of proteins. Erythromycin may be bacteriostatic or bactericidal depending on the organism and drug concentration https://www.drugbank.ca/
Actinomycin D: prevents transcription of Bacterial DNA; induces apoptosis; Rotaviruses are the major cause of viral diarrhea in humans and animals. Actinomycin D (Act D) is an antibiotic that intercalates DNA and therefore inhibits DNA-dependent transcription. The current study was carried out to assess the influence of Act D on the replication of simian rotavirus (SA11) in cell culture. Virus-infected MA-104 cell cultures were studied in the presence of Act D at concentrations of 1.25 and 2.5 µg/ml. Treatment of rotavirus-infected cells with 2.5 µg/ml Act D 48 h post-infection reduced the cytoplasmic metachromasia after staining with acridine orange by 25%. Viral RNA labeled with 3H-uridine in the presence of the drug was separated by polyacrylamide gel electrophoresis. Viral RNA replication was not affected by Act D, but increased 3H-uridine uptake was demonstrable by infected cells in the presence of the drug. This possibly was due to the inhibition of cellular RNA synthesis by Act D, which thus enhances incorporation of the radionuclide into the viral RNA. Act D reduced the number of infected cells presenting virus-specific fluorescence 48 h post-infection by more than 50%. These data suggest that Act D may have complexed with viral RNA and prevented newly synthesized mRNA from being translated, but may not have prevented early replication. ActD CTG of ATGCTGCAT sequence binds to a DNA sequence (CTG)n tiplets; it is a potential anticancer drug. https://www.therm
Quinolone : Bactericidal Antibiotic-induced cell death has been associated with the formation of double-stranded DNA breaks following treatment with DNA gyrase inhibitors. Modulation of chromosomal supercoiling through topoisomerase-catalyzed strand breakage and rejoining reactions is required for DNA synthesis, mRNA transcription and cell division; synthetic quinolone class of antimicrobials, including the clinically-relevant fluoroquinolones, which target DNA-topoisomerase complexes; Quinolones are derivatives of Nalidixic acid, Synthetic quinolone- (floroquinolones) binds to DNA topoisomerases and prevents broken DNA strand repair by inhibiting Gyrase A and Topoisomerase IV of Salmonella typhimurium- Nalidixic acid_acts on bacteria like Salmonella by inhibiting Gyrase A and Topoisomerase IV of Salmonella typhimurium http://link.springer. Quinolones and derivatives, have also been isolated from natural sources (such as plants, animals and bacteria) and can act as natural antimicrobials and/or signalling molecules, prevent opening of dsDNA at the time of transcription; Due to sickle-cell disease patients' being at increased risk for developing osteomyelitis from the Salmonella genus, fluoroquinolones are the "drugs of choice" due to their ability to enter bone tissue without chelating it, as tetracyclines are known to do.
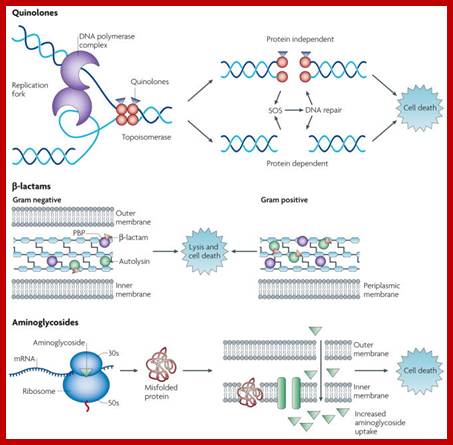
Drug-target interactions and associated cell death mechanisms; http://link.springer.
Together with studies revealing that co-treatment with quinolones and the protein synthesis inhibitor, chloramphenicol, inhibits the ability of certain quinolones to kill bacteria-NOTE
Cycloheximide (CHI); Produced by Streptomyces griseus- by binding to 80s ribosomes prevents protein synthesis-inhibiting translocation of two tRNAs- it is so toxic it is used only in vtro labworks.-Treatment leads to apoptosis. CHI induces UBI4 polyubiqutin gene. We report here on the development and validation of the dynamic protein synthesis translocation reporter (dPSTR), that provides real-time measurement of protein expression arising from a promoter element in live single cells. For example, homo harringtonine (Omacetaxine) was screened as an alkaloid with anti-tumoral properties and was shown later to affect protein synthesis, it now has become the first approved drug against chronic myelogenous leukaemia; A crystal structure of CHX bound to the yeast ribosome has revealed the location of the binding site on the ribosome suggesting that CHX and the 3′ CCA end of the exit (E) site transfer RNA (tRNA) share a common binding region at the E-site; ligand-induced active release of the E-site tRNA- tRNA from P site to E site
Macrolides, streptogramins, spectinomycin, tetracycline, chloramphenicol and macrolides are typically bacteriostatic, however, these families of ribosome inhibitors can be bactericidal in a species- or treatment-specific fashion. For example, chloramphenicol has been shown to kill S. pneumoniae and Neisseria meningitides effectively, while chloramphenicol and the macrolide, azithromycin, have exhibited bactericidality against Haemophilus influenza.
Binding of aminoglycosides to the ribosome does not bring translation to an immediate standstill. Rather, as noted above, this class of drugs promotes protein mistranslation through the incorporation of inappropriate amino acids into elongating peptide strands, a phenotype specific for aminoglycosides and one which contributes to cell killing.
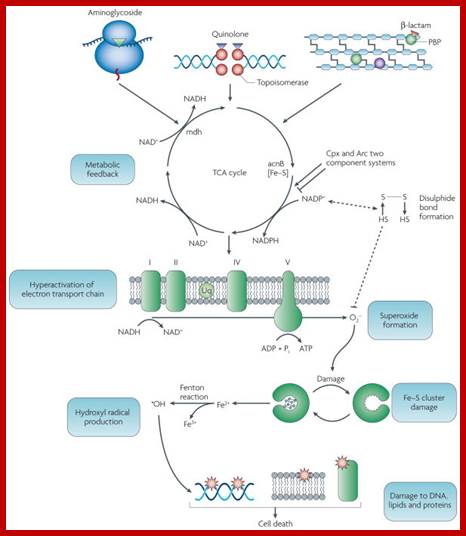
Common mechanism of cell death induced by bactericidal antibiotics; https://www.ncbi.nlm.nih.gov/
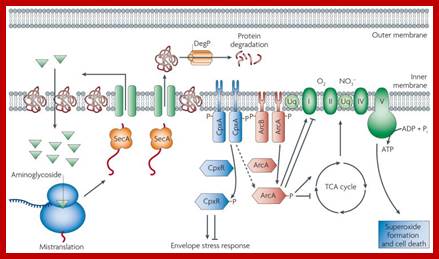
Aminoglycosides trigger for radical-mediated cell death; https://www.ncbi.nlm.nih.gov/
Tetracyclines: Inhibit protein synthesis by binding to 30S ribosomal subunit and prevent the binding of aatRNA, thus inhibit their steps in translation.
Erythromycin; Bacterial infections; including syphilis, respiratory tract infections, skin infections, chlamydia infections, and syphilis. Available in the form of capsules, tablets.
Erythromycin is metabolized by enzymes of the cytochrome P450 system, in particular, by isozymes of the CYP3A superfamily, CYP3A; Erythromycin displays bacteriostatic activity or inhibits growth of bacteria, especially at higher concentrations, but the mechanism is not fully understood. By binding to the 50s subunit of the bacterial rRNA complex, protein synthesis and subsequent structure and function processes critical for life or replication are inhibited; Erythromycin interferes with aminoacyl translocation, preventing the transfer of the tRNA bound at the A site of the rRNA complex to the P site of the rRNA complex. Most of erythromycin is metabolized by demethylation in the liver by the hepatic enzyme CYP3A4. Standard-grade erythromycin is primarily composed of four related compounds known as erythromycins A, B, C, and D.

Chloromycetin:
It works on many disease causing microbes that causes meningitis, plague, cholera, and typhoid fever. Its use is only recommended when safer antibiotics cannot be used; Common side effects include bone marrow suppression, nausea, and diarrhea. It is the first-choice treatment for staphylococcal brain abscesses; meningitis: Neisseria meningitidis, Streptococcus pneumoniae, and Haemophilus influenzae. It can cause- Bonemarrow suppression, Leukemia, Gray baby syndrome, Hypersensitivity
It binds to bacterial ribosomal subunit and prevents protein chain elongation by inhibiting the peptidyl transferase activity of the bacterial ribosome. It specifically binds to A2451 and A2452 residues in the 23S rRNA of the 50S ribosomal subunit, preventing peptide bond formation. While chloramphenicol and the macrolide class of antibiotics both interact with ribosomes, chloramphenicol is not a macrolide. It directly interferes with substrate binding, whereas macrolides sterically block the progression of the growing peptide.
Tetracyclines:
It is well established that tetracyclines inhibit bacterial protein synthesis by preventing the association of aminoacyl-tRNA with the bacterial ribosome .
Several studies have indicated a single, high-affinity binding site for tetracyclines in the ribosomal 30S subunit, with indications through photoaffinity labeling and chemical footprinting studies that protein S7 and 16S rRNA bases G693, A892, U1052, C1054, G1300, and G1338 contribute to the binding pocket ; Tetracycline (which measures approximately 8 by 12 Å) to the ribosome appears to cause wide-ranging structural change in 16 S rRNA.
Antiviral:
|
Acyclovir |
Zovirax, Sitavig |
|
Brivudine |
Helpin |
|
Docosanol |
Abreva |
|
Famciclovir |
Famvir |
|
Foscarnet |
Foscavir |
|
Idoxuridine |
Herplex, Dendrid |
|
Penciclovir |
Denavir |
|
Trifluridine |
Viroptic |
|
Valacyclovir |
Valtrex |
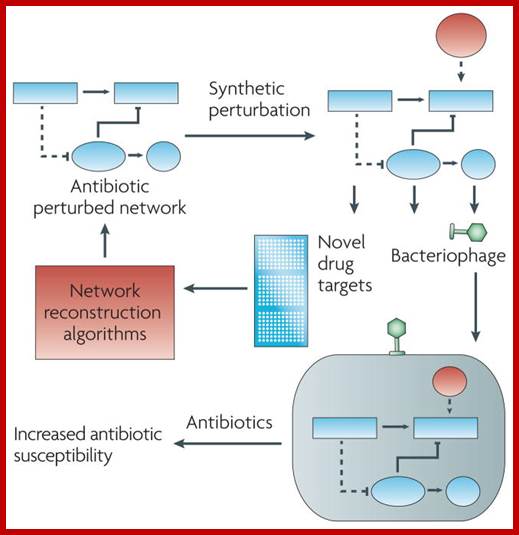
Network for antibiotic functional analysis and therapeutic development; https://www.ncbi.nlm.nih.gov
Antiviral:
|
Amantadine |
Symmetrel |
|
Rimantadine |
Flumadine |
|
Neuraminidase Inhibitors |
|
|
Oseltamivir |
Tamiflu |
|
Peramivir |
Rapivab |
|
Zanamivir |
|
Antihelmentic
|
Bephenium |
|
|
Diethylcarbamazine |
|
|
Ivermectin |
Sklice, Stromectol, Ivomec, Mectizan |
|
Niclosamide |
Niclocide |
|
Piperazine |
|
|
Praziquantel |
Biltricide |
|
Pyrantel |
Antiminth, Aut, Cobantril, Helmex, Lombriareu, Trilombrin |
|
Pyrvinium |
Vanuin, Viprynium |
|
Benzimidazoles |
|
|
Albendazole |
Albenza |
|
Flubendazole |
Fluvermal |
|
Mebendazole |
Mebendacin, Mebutar, Nemazole, Vermox |
|
Thiabendazole |
|
Non-Steroidal Anti-Inflammatory Drugs (NSAIDs);
|
Generic |
Brand Name |
|
Bromfenac |
Prolensa, Bromday |
|
Diclofenac |
Cataflam, Voltaren, Zipsor |
|
Diflunisal |
Dolobid |
|
Etodolac |
Lodine, Lodine XL |
|
Fenoprofen |
Nalfon |
|
Flurbiprofen |
Ansaid |
|
Ibuprofen |
Advil, Cramp End, Dolgesic, Excedrin IB, Genpril, Haltran, Ibren, Ibu, Ibuprin, Ibuprohm, Ibu-Tab, Medipren, Midol IB, Motrin, Nuprin, Pamprin-IB, Q-Profen, Rufen, Trendar |
|
Indomethacin |
Indocin, Indocin SR, Tivorbex |
|
Ketoprofen |
Actron, Orudis, Oruvail |
|
Ketorolac |
Toradol, Sprix |
|
Meclofenamate |
Meclomen |
|
Mefenamic Acid |
Ponstel |
|
Meloxicam |
Mobic, Vivlodex |
|
Nabumetone |
Relafen |
|
Naproxen |
Aleve, Anaprox, |
|
Nepafenac |
Nevanac |
|
Oxaprozin |
Daypro |
|
Phenylbutazone |
Cotylbutazone |
|
Piroxicam |
Feldene |
|
Sulindac |
Clinoril |
|
Tolmetin |
Tolectin, Tolectin DS |
Toremifene- Tamoxifen and raloxifene are commonly used selective estrogen receptor modulators for treatment of breast cancer in women with high risk, although resistance occurs by tamoxifen after 5 years of therapy and both drugs cause uterine cancer and thromboembolic events. Aromatase inhibitors (AIs) are one of the optional modes used for breast cancer treatment. The combination of AIs along with tamoxifen can also be beneficial. https://www.ncbi.nlm.nih.gov Shazia Ali,1 Neelima Mondal,et al
Ribozyme: Ribozymes (ribonucleic acid enzymes) are RNA molecules that are capable of catalyzing specific biochemical reactions, similar to the action of protein enzymes. The 1982 discovery of ribozymes demonstrated that RNA can be both genetic material (like DNA) and a biological catalyst (like protein enzymes), and contributed to the RNA world hypothesis, which suggests that RNA may have been important in the evolution of prebiotic self-replicating systems.
The most common activities of natural or in vitro-evolved ribozymes are the cleavage or ligation of RNA and DNA and peptide bond formation; Within the ribosome, ribozymes function as part of the large subunit ribosomal RNA to link amino acids during protein synthesis. They also participate in a variety of RNA processing reactions, including RNA splicing, viral replication, and transfer RNA biosynthesis. Examples of ribozymes include the hammerhead ribozyme, the VS ribozyme, Leadzyme and the hairpin ribozyme.
https://en.wikipedia.org; https://en.wikipedia.org
3D structure of a hammerhead ribozyme; Full-Length Hammerhead Ribozyme color-coded so that the 5'-end of each RNA strand is blue and the 3'-end is red. The individual nucleotides are represented as toothpicks, and the phosphodiester backbone as a narrow tube.
Investigators studying the origin of life have produced ribozymes in the laboratory that are capable of catalyzing their own synthesis from activated monomers under very specific conditions, such as an RNA polymerase ribozyme.[2] Mutagenesis and selection has been performed resulting in isolation of improved variants of the "Round-18" polymerase ribozyme from 2001. "B6.61" is able to add up to 20 nucleotides to a primer template in 24 hours, until it decomposes by cleavage of its phosphodiester bonds.[3] The "tC19Z" ribozyme can add up to 95 nucleotides with a fidelity of 0.0083 mutations/nucleotide
Such RNA ribozymes were found in the intron of an RNA transcript, which removed itself from the transcript, as well as in the RNA component of the RNase P complex, which is involved in the maturation of pre-tRNAs. In 1989, Thomas R. Cech and Sidney Altman shared the Nobel Prize in chemistry for their "discovery of catalytic properties of RNA." The term ribozyme was first introduced by Kelly Kruger et al. in 1982 in a paper published in Cell.
Losartan-is a startan type of medicine;
Angiotensin receptor blockers (ARB) or it is deemed as angiotensin receptor antagonists (ARA). Angiotensin II receptor antagonists, also known as angiotensin receptor blockers (ARBs), AT1-receptor antagonists or sartans, are a group of pharmaceuticals that modulate the renin–angiotensin system. Their main uses are in the treatment of hypertension (high blood pressure), diabetic nephropathy (kidney damage due to diabetes) and congestive heart failure. They block activation of angiotensin II AT1 receptors, preventing angiotensin II from binding there. Losartan works by blocking the angiotensin II hormone from binding to a receptor in the body, which normally leads to certain changes that increase the blood pressure. It is a nonpeptide receptor angiotensin II type 1 (AT1)–selective, nonpeptide receptor antagonist. Candesartan,Eprosartan, Irbesartan, Olmesartan, Telmisartan, Valsartan. It is a nonpeptide receptor angiotensin II type 1 (AT1)–selective, nonpeptide receptor antagonist
TB drug-
Bedaquiline; http://www.tbfacts.org/
Ethionamide , kanamycin, pyrazinamide, Afloxacin, cycloserine/terizidone or an available alternative. The most common side effects are headache, dizziness, feeling sick, being sick, joint pain and increases in liver enzymes. Side effects can be experienced by more than one in ten people. For high income countries the situation is very different, with bedaquiline costing US$30,000 in the United States. This is equivalent to £18,800 in the UK -
Dosage;The recommended dose is 400 mg a day for two weeks and then 200 mg taken three times a week (with at least 48 hours between doses) for the next 22 weeks. Bedaquiline is available as 100 mg tablets, and the tablets should be swallowed whole with water and taken with food. - See more at: Other drugs-ethionamide kanamycin pyrazinamide ofloxacin cycloserine/terizidone or an available alternative. - See more at: New drug First FDA approved TB drug for 40 years. For high income countries the situation is very different, with bedaquiline costing US$30,000 in the United States. The drug will be available as part of second line treatment for patients suffering from MDR-TB and XDR-TB. - See more at: http://www.tbfacts.org/bedaquiline/#sthash.Mh3AWX1j.dpuf

See more at: http://www.tbfacts.org/bedaquiline/#sthash.Mh3AWX1j.dpuf
Bedaquiline also called Sirturo is the active substance in the TB drug Sirturo; Bedaquiline works by blocking an enzyme inside the Mycobacterium tuberculosis bacteria called ATP synthase. This enzyme is used by the bacteria to generate energy. Without the ability to generate energy, the TB bacteria die and the patient’s condition can start to improve. Bedaquiline was recently approved for the treatment of multidrug-resistant tuberculosis (MDR-TB).
Bedaquiline is an adenosine triphosphate (ATP) synthase inhibitor specific for MTB and some nontuberculous mycobacteria. Its chemical name is 1-(6-bromo-2methoxy-quinolin-3-yl)-4-dimethylamino-2-naphtalen-1-yl-1-phenyl-butan-2-ol.
Bedaquiline kills both dormant and actively replicating mycobacteria by inhibiting mycobacterial adenosine triphosphate (ATP) synthase, an essential membrane-bound enzyme, interfering with energy production and disrupting intracellular metabolism; Evidence suggests that it acts by interfering with the proton transfer chain [de Jonge et al. 2007]. Inhibition of ATP synthase by bedaquiline is specific for mycobacteria. It demonstrated considerably higher minimum inhibitory concentrations (MIC) against gram positive and gram negative bacteria [Andries et al. 2005]. Bedaquiline does not interfere with mammalian ATP synthase activity. Human mitochondrial ATP synthase sensitivity to bedaquiline was 20,000-fold less than the mycobacterial enzyme, See more at: http://www.tbfacts.org/bedaquiline/#sthash.m26CCKmG.dpuf
Antibiotics: Ofloxacin and Ornidazole
In order to understand treatment with the combination therapy of these two drugs, let’s take a look first at each of them individually to understand what each is used for:
Ofloxacin
Ofloxacin is a fluoroquinolone antibiotic used to fight off bacterial infections in your body. This type of antibiotic treats things like bladder infections, kidney infections, skin, lung, and prostate infections. It is also used in ear infections. Other conditions it treats are chlamydia, gonorrhea, and pelvic inflammatory disease. There are other uses “off label” that Ofloxacin is used for, including Tuberculosis (not approved by the FDA), corneal inflammation when given before eye surgery, and mycoplasma pneumonia.
Ornidazole
This medication is used for certain infections that are caused byanaerobic bacteria (not requiring oxygen), amoebic, and parasitic infections. These infections are commonly found in the urinary tract, vagina, and the intestines. The antibiotic may also be given to prevent these types of infections.
Some of the common infections this medication treats are amoebic dysentery, bacterial vaginosis, Trichomoniasis, giardiasis, hepatic amoebas, and off-label for Crohn’s Disease.
Ofloxacin and Ornidazole Combination Uses;
· Doctors use a combination of Ofloxacin and Ornidazole for infections in the digestive tract that lead to abdominal pain and diarrhea. This combination treats amoebic and protozoan infections.
· It also treats parasitic and fungal infections in oral cavity or vagina.
· The combination is often very effective when you have a urinary tract infection.
· It is also helpful to use this combination when respiratory infections are caused by very aggressive bacteria like pneumonia and bronchitis. The doctor may use this medication in a “short burst” over the course of 3 to 5 days.
· It is also helpful in bringing some sexually transmitted diseases (STD) under control. Severe cases of Chlamydia and Gonorrhea can be treated in short courses with high doses of this combination to eradicate the infection quickly. Some cases of STD’s fail therapy because the patient stops taking the antibiotics. This combination can work to clear these infections in just a few doses. This eliminates the chance of reoccurrence or drug resistance because the course of treatment is easier and faster to complete.
· Leprosy, also known as “Hansen’s Disease” is caused by a very hard to kill bacteria. It was once very contagious, but combination therapies like Ofloxacin and Ornidazole can help eradicate the infection. Sometimes this combination is combined with other drugs to shorten the time of treatment and can reduce the risk of transmission of Leprosy to others.
· Dasage for Oflotxacin and ornidazole: Adults: The combination tablets of Ofloxacin and Ornidazole come in a single dose tablet containing 200mg of Ofloxacin and 500mg Ornidazole.
The usual adult dosage is: One tablet taken twice daily for 5 to 10 days depending on the severity of the infection.
Human epidermal growth factor (940)[EGF}; stimulates cell growth, proliferation, and differentiation by binding to its receptor EGFR. Human EGF is a 6045-Da protein[3] with 53 amino acid residues and human unrine. Salivary EGF, which seems also regulated by dietary inorganic iodine, also plays an important physiological role in the maintenance of oro-esophageal and gastric tissue integrity. EGF acts by binding with high affinity to epidermal growth factor receptor (EGFR) on the cell surface. This stimulates ligand-induced dimerization,[9] activating the intrinsic protein-tyrosine kinase activity of the receptor (see the second diagram). The tyrosine kinase activity, in turn, initiates a signal transduction cascade that results in a variety of biochemical changes within the cell – a rise in intracellular calcium levels, increased glycolysis and protein synthesis, and increases in the expression of certain genes including the gene for EGFR – that ultimately lead to DNA synthesis and cell proliferation
EGF is the founding member of the EGF-family of proteins. Members of this protein family have highly similar structural and functional characteristics. Besides EGF itself other family members include: Heparin-binding EGF-like growth factor (HB-EGF) EGF family:
https://en.wikipedia.org Diagram showing key components of the MAPK/ERK pathway. In the diagram, "P" represents phosphate. Note EGF at the very top. Recombinant human epidermal growth factor, sold under the brand name Heberprot-P, is used to treat diabetic foot ulcers. It can be given by injection into the wound site,[15] or may be used topically.[16] Tentative evidence shows improved wound healing.[17] Safety has been poorly studied
Human granulocyte colony stimulating factor (G-CSF or GCSF) (950); Granulocyte-colony stimulating factor (G-CSF or GCSF), also known as colony-stimulating factor 3 (CSF 3), is a glycoprotein that stimulates the bone marrow to produce granulocytes and stem cells and release them into the bloodstream. Functionally, it is a cytokine and hormone, a type of colony-stimulating factor, and is produced by a number of different tissues. The pharmaceutical analogs of naturally occurring G-CSF are called filgrastim and lenograstim. G-CSF also stimulates the survival, proliferation, differentiation, and function of neutrophil precursors and mature neutrophils.
G-CSF is produced by endothelium, macrophages, and a number of other immune cells. The natural human glycoprotein exists in two forms, a 174- and 177-amino-acid-long protein of molecular weight 19,600 grams per mole. The more-abundant and more-active 174-amino acid form has been used in the development of pharmaceutical products by recombinant DNA (rDNA) technology.
White blood cells: The G-CSF-receptor is present on precursor cells in the bone marrow, and, in response to stimulation by G-CSF, initiates proliferation and differentiation into mature granulocytes. G-CSF stimulates the survival, proliferation, differentiation, and function of neutrophil precursors and mature neutrophils. G-CSF regulates them using Janus kinase (JAK)/signal transducer and activator of transcription (STAT) and Ras/mitogen-activated protein kinase (MAPK) and phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt) signal transduction pathway.
The gene for G-CSF is located on chromosome 17, locus q11.2-q12. Nagata et al. found that the GCSF gene has 4 introns, and that 2 different polypeptides are synthesized from the same gene by differential splicing of mRNA;
The gene for G-CSF is located on chromosome 17, locus q11.2-q12. Nagata et al. found that the GCSF gene has 4 introns, and that 2 different polypeptides are synthesized from the same gene by differential splicing of mRNA.
The 2 polypeptides differ by the presence or absence of 3 amino acids. Expression studies indicate that both have authentic GCSF activity. It is thought that stability of the G-CSF mRNA is regulated by an RNA element called the G-CSF factor stem-loop destabilizing element. The recombinant human G-CSF (rhG-CSF) synthesized in an E. coli expression system is called Filgrastim. The structure of filgrastim differs slightly from the structure of the natural glycoprotein. Most published studies have used filgrastim.
Filgrastim was first marketed by Amgen with the brand name Neupogen. Several bio-generic versions are now also available in markets such as Europe and Australia. Filgrastim (Neupogen) and PEG-filgrastim (Neulasta) are two commercially-available forms of rhG-CSF. The PEG (polyethylene glycol) form has a much longer half-life, reducing the necessity of daily injections.
Another form of rhG-CSF called lenograstim is synthesised in Chinese Hamster Ovary cells (CHO cells). As this is a mammalian cell expression system, lenograstim is indistinguishable from the 174-amino acid natural human G-CSF. No clinical or therapeutic consequences of the differences between filgrastim and lenograstim have yet been identified, but there are no formal comparative studies.
Human growth hormone (960): http://pdb101.rcsb.org/motm/52: Growth hormone is one key growth signal released from the pituitary, a pea-sized gland located at the base of the brain. Lack of this hormone in children can cause them to remain shorter than average, while in its excess they may grow taller than most. Growth hormone continues its work in adults. Growth hormone travels through the blood and stimulates the liver to produce a protein called insulin-like growth factor (IGF-1), shown at the bottom here using coordinates in PDB entry 1h02 . IGF-1 helps the cartilage cells located at the ends of long bones to multiply. In children, this leads to growth in the length of the bones and increases the child's height. By puberty, however, the cartilage at the ends of most long bones is converted to bone and subsequent action of growth hormone or IGF-1 usually cannot increase their length. IGF-1 also acts on immature muscle cells to increase muscle mass. Aside from these growth stimulating functions, growth hormone participates in regulating the body's metabolism. It acts on fat cells to reduce the amount of stored fats, promotes protein synthesis in cells and plays a role in regulating the sugar levels in the blood. Thus growth hormone has multiple effects on the overall form and function of a growing body. By mid to late 1980s scientists were able to produce this 191-residue protein hormone in bacteria using recombinant DNA technology. With the availability of large quantities of this recombinant hormone, therapeutic uses of growth hormone became possible. Children and adults with growth hormone deficiency can now be treated with growth hormone supplements. Growth Hormone in Action
Growth hormone (shown here in red) performs its multiple functions by binding to growth hormone receptors (shown here in blue and green) on its target organs and cells. These receptors have separate portions outside and inside the cell, connected by a helical stretch that passes through the cell membrane. Interestingly, growth hormone must bind to two receptor molecules simultaneously to mediate its function. The hormone binds on the outside of the cell, bringing two receptors together. PDB entry 3hhr , shown here, includes the extracellular portion of two receptors bound to growth hormone. When two receptors are brought together, interaction between the portions inside the cell (shown here schematically) triggers several enzymatic reactions and signaling processes that stimulate growth. Thus formation of this receptor dimer is crucial for growth hormone function. One of the major surprises from this crystal structure was the discovery that the two receptor molecules, which are structurally identical, bind to two structurally distinct sites on opposite sides of a single growth hormone molecule. As you might expect, the strength of binding at these two sites is also different.
Receptor for growth hormone. http://pdb101.rcsb.org/motm/52
The hormone is shown in red, and the portions of the receptor crossing the membrane, which are not included in the structure, are shown schematically.
Two growth hormone receptors bind to the hormone one after the other. Binding to the first site forms an inactive intermediate complex. The assembly becomes functionally active only when the second receptor molecule binds at the second binding site on the other side of the hormone. Any factor that disrupts this assembly can block the hormone's function. For example, changing a single glycine at position 120 to arginine will block receptor binding at the second site. By comparing the altered hormone (shown on the right from PDB entry 1hwh ) with the normal hormone (shown on the left from PDB entry 1hwg ), you can see that the large side chain of arginine 120 cannot be accommodated when the second receptor binds. This residue clashes with tryptophan 104 of the receptor (shown as green spheres). Thus the altered hormone cannot form a functional complex with a second receptor and no longer promotes growth - in fact it suppresses growth.
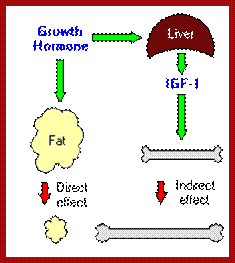
http://arbl.cvmbs.colostate.edu;
- Direct effects are the result of growth hormone binding its receptor on target cells. Fat cells (adipocytes), for example, have growth hormone receptors, and growth hormone stimulates them to break down triglyceride and supresses their ability to take up and accumulate circulating lipids.
- Indirect effects are mediated primarily by a insulin-like growth factor-I (IGF-I), a hormone that is secreted from the liver and other tissues in response to growth hormone. A majority of the growth promoting effects of growth hormone is actually due to IGF-I acting on its target cells.
- It effects Protein metabolism, fat metabolism and carbohydrate metabolism.
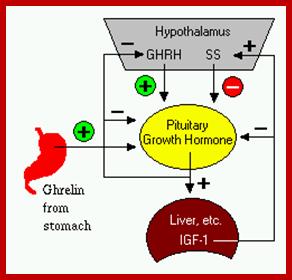
http://arbl.cvmbs.colostate.edu/
Control of Growth Hormone Secretion
Production of growth hormone is modulated by many factors, including stress, exercise, nutrition, sleep and growth hormone itself. However, its primary controllers are two hypothalamic hormones and one hormone from the stomach:
- Growth hormone-releasing hormone (GHRH) is a hypothalamic peptide that stimulates both the synthesis and secretion of growth hormone.
- Somatostatin (SS) is a peptide produced by several tissues in the body, including the hypothalamus. Somatostatin inhibits growth hormone release in response to GHRH and to other stimulatory factors such as low blood glucose concentration.
- Ghrelin is a peptide hormone secreted from the stomach. Ghrelin binds to receptors on somatotrophs and potently stimulates secretion of growth hormone.
Growth hormone secretion is also part of a negative feedback loop involving IGF-I. High blood levels of IGF-I lead to decreased secretion of growth hormone not only by directly suppressing the somatotroph, but by stimulating release of somatostatin from the hypothalamus.
Growth hormone (GH) is widely used as a performance-enhancing drug. One of its best-characterized effects is increasing levels of circulating insulin-like growth factor I (IGF-I), which is primarily of hepatic origin. It also induces synthesis of IGF-I in most non-hepatic tissues. The effects of GH in promoting postnatal body growth are IGF-I dependent, but IGF-I-independent functions are beginning to be elucidated. Although benefits of GH administration have been reported for those who suffer from GH deficiency, there is currently very little evidence to support an anabolic role for supra-physiological levels of systemic GH or IGF-I in skeletal muscle of healthy individuals. There may be other performance-enhancing effects of GH. In contrast, the hypertrophic effects of muscle-specific IGF-I infusion are well documented in animal models and muscle cell culture systems. Studies examining the molecular responses to hypertrophic stimuli in animals and humans frequently cite upregulation of IGF-I messenger RNA or immunoreactivity. The circulatory/systemic (endocrine) and local (autocrine/paracrine) effects of GH and IGF-I may have distinct effects on muscle mass regulation.
Growth hormone is a 191-amino acid protein, single-chain polypeptide that is synthesized, stored, and secreted by somatotropic cells within the lateral wings of the anterior pituitary gland. The major isoform of the human growth hormone is a protein of 191 amino acids and a molecular weight of 22,124 daltons. The structure includes four helices necessary for functional interaction with the GH receptor.
Several molecular isoforms of GH exist in the pituitary gland and are released to blood. In particular, a variant of approximately 20 kDa originated by an alternative splicing is present in a rather constant 1:9 ratio,[9] while recently an additional variant of ~ 23-24 kDa has also been reported in post-exercise states at higher proportions.[10] This variant has not been identified, but it has been suggested to coincide with a 22 kDa glycosylated variant of 23 kDa identified in the pituitary gland.[11] Furthermore, these variants circulate partially bound to a protein (growth hormone-binding protein, GHBP), which is the truncated part of the growth hormone receptor, and an acid-labile subunit (ALS).
Secretion of growth hormone (GH) in the pituitary is regulated by the neurosecretory nuclei of the hypothalamus. These cells release the peptides Growth hormone-releasing hormone (GHRH or somatocrinin) and Growth hormone-inhibiting hormone (GHIH or somatostatin) into the hypophyseal portal venous blood surrounding the pituitary. GH release in the pituitary is primarily determined by the balance of these two peptides, which in turn is affected by many physiological stimulators (e.g., exercise, nutrition, sleep) and inhibitors (e.g., free fatty acids) of GH secretion
Effects of growth hormone on the tissues of the body can generally be described as anabolic (building up). Like most other protein hormones, GH acts by interacting with a specific receptor on the surface of cells.
Increased height during childhood is the most widely known effect of GH. Height appears to be stimulated by at least two mechanisms:
1. Because polypeptide hormones are not fat-soluble, they cannot penetrate cell membranes. Thus, GH exerts some of its effects by binding to receptors on target cells, where it activates the MAPK/ERK pathway. Through this mechanism GH directly stimulates division and multiplication of chondrocytes of cartilage.
2. GH also stimulates, through the JAK-STAT signaling pathway, the production of insulin-like growth factor 1 (IGF-1, formerly known as somatomedin C), a hormone homologous to proinsulin. The liver is a major target organ of GH for this process and is the principal site of IGF-1 production. IGF-1 has growth-stimulating effects on a wide variety of tissues. Additional IGF-1 is generated within target tissues, making it what appears to be both an endocrine and an autocrine/paracrine hormone. IGF-1 also has stimulatory effects on osteoblast and chondrocyte activity to promote bone growth.
In addition to increasing height in children and adolescents, growth hormone has many other effects on the body:
· Increases calcium retention, and strengthens and increases the mineralization of bone.
· Increases muscle mass through sarcomere hypertrophy
· Promotes lipolysis
· Increases protein synthesis
· Stimulates the growth of all internal organs excluding the brain
· Plays a role in homeostasis
· Reduces liver uptake of glucose
· Promotes gluconeogenesis in the liver[37]
· Contributes to the maintenance and function of pancreatic islets
· Stimulates the immune system
Increases deiodination of T4 to T3.
Antibiotics: Ofloxacin and Ornidazole
In order to understand treatment with the combination therapy of these two drugs, let’s take a look first at each of them individually to understand what each is used for:
Ofloxacin
Ofloxacin is a fluoroquinolone antibiotic used to fight off bacterial infections in your body. This type of antibiotic treats things like bladder infections, kidney infections, skin, lung, and prostate infections. It is also used in ear infections. Other conditions it treats are chlamydia, gonorrhea, and pelvic inflammatory disease. There are other uses “off label” that Ofloxacin is used for, including Tuberculosis (not approved by the FDA), corneal inflammation when given before eye surgery, and mycoplasma pneumonia.
Ornidazole
This medication is used for certain infections that are caused byanaerobic bacteria (not requiring oxygen), amoebic, and parasitic infections. These infections are commonly found in the urinary tract, vagina, and the intestines. The antibiotic may also be given to prevent these types of infections.
Some of the common infections this medication treats are amoebic dysentery, bacterial vaginosis, Trichomoniasis, giardiasis, hepatic amoebas, and off-label for Crohn’s Disease.
Ofloxacin and Ornidazole Combination Uses;
· Doctors use a combination of Ofloxacin and Ornidazole for infections in the digestive tract that lead to abdominal pain and diarrhea. This combination treats amoebic and protozoan infections.
· It also treats parasitic and fungal infections in oral cavity or vagina.
· The combination is often very effective when you have a urinary tract infection.
· It is also helpful to use this combination when respiratory infections are caused by very aggressive bacteria like pneumonia and bronchitis. The doctor may use this medication in a “short burst” over the course of 3 to 5 days.
· It is also helpful in bringing some sexually transmitted diseases (STD) under control. Severe cases of Chlamydia and Gonorrhea can be treated in short courses with high doses of this combination to eradicate the infection quickly. Some cases of STD’s fail therapy because the patient stops taking the antibiotics. This combination can work to clear these infections in just a few doses. This eliminates the chance of reoccurrence or drug resistance because the course of treatment is easier and faster to complete.
· Leprosy, also known as “Hansen’s Disease” is caused by a very hard to kill bacteria. It was once very contagious, but combination therapies like Ofloxacin and Ornidazole can help eradicate the infection. Sometimes this combination is combined with other drugs to shorten the time of treatment and can reduce the risk of transmission of Leprosy to others.
· Dasage for Oflotxacin and ornidazole: Adults: The combination tablets of Ofloxacin and Ornidazole come in a single dose tablet containing 200mg of Ofloxacin and 500mg Ornidazole.
The usual adult dosage is: One tablet taken twice daily for 5 to 10 days depending on the severity of the infection.
Human adenosine deaminase; The ADA gene provides instructions for producing the enzyme adenosine deaminase. This enzyme is produced in all cells, but the highest levels of adenosine deaminase occur in immune system cells called lymphocytes, which develop in lymphoid tissues. These lymphoid tissues include the thymus, which is a gland located behind the breastbone, and lymph nodes, which are found throughout the body. Lymphocytes form the immune system, which defends the body against potentially harmful invaders, such as viruses or bacteria.
The function of the adenosine deaminase enzyme is to eliminate a molecule called deoxyadenosine, which is generated when DNA is broken down. Adenosine deaminase converts deoxyadenosine, which is toxic to lymphocytes, to another molecule called deoxyinosine, which is not harmful. Adenosine Deaminase (also known as adenosine aminohydrolase, or ADA) is an enzyme (EC 3.5.4.4) involved in purine metabolism. It is needed for the breakdown of adenosine from food and for the turnover of nucleic acids in tissues. Its primary function in humans is the development and maintenance of the immune system.
![]()
https://en.wikipedia.org; https://ghr.nlm.nih.gov
The ADA active site contains a zinc ion, which is located in the deepest recess of the active site and coordinated by five atoms from His15, His17, His214, Asp295, and the substrate.[4] Zinc is the only cofactor necessary for activity.
Difficiency-Causes severe combined immune deficiency; Adenosine deaminase (ADA) is an enzyme involved in purine metabolism and is essential for lymphocyte development, survival, and function. ADA deficiency is an autosomal-recessive inherited disorder that can result in severe combined immunodeficiency (SCID). Affected infants with ADA-deficient SCID usually are diagnosed by 6 months of age, after presenting with failure to thrive and recurrent opportunistic infections, because of profound pan-lymphopenia and the virtual absence of humoral and cellular immunity. ADA-deficient SCID is almost always fatal by 2 years of age if immunity is not restored
http://fbme.utm.my/ http://fbme.utm.my/Fabio Candotti, et al 30 and odd.
Tissue plasminogen activator:
It is a serine protease (EC 3.4.21.68) found on endothelial cells, the cells that line the blood vessels. As an enzyme, it catalyzes the conversion of plasminogen to plasmin, the major enzyme responsible for clot breakdown. Because it works on the clotting system, tPA (such as alteplase, reteplase, and tenecteplase) is used in clinical medicine to treat embolic or thrombotic stroke. Use is contraindicated in hemorrhagic stroke and head trauma. The antidote for tPA in case of toxicity is aminocaproic acid.
tPA may be manufactured using recombinant biotechnology techniques. tPA created this way may be referred to as recombinant tissue plasminogen activator (rtPA). It is sold as alteplase. Medical uses; tPA is used in some cases of diseases that feature blood clots, such as pulmonary embolism, myocardial infarction, and stroke, in a medical treatment called thrombolysis. The most common use is for ischemic stroke. It can either be administered systemically, in the case of acute myocardial infarction, acute ischemic stroke, and most cases of acute massive pulmonary embolism, or administered through an arterial catheter directly to the site of occlusion in the case of peripheral arterial thrombi and thrombi in the proximal deep veins of the leg.
It has been suggested that if tPA is effective in ischemic stroke, it must be administered as early as possible after the onset of stroke symptoms. Recombinant tissue plasminogen activators (r-tPAs) include alteplase, reteplase, and tenecteplase (TNKase). https://en.wikipedia.org
Tissue plasminogen activator (tPA), tissue-type plasminogen activator) is a serine protease found on endothelial cells (cells that line the blood vessels) involved in the breakdown of blood clots (fibrinolysis). tPA enzyme catalyzes the conversion of plasminogen to plasmin.
tPA may be manufactured using recombinant biotechnology techniques. tPA created this way may be referred to as recombinant tissue plasminogen activator (rtPA).
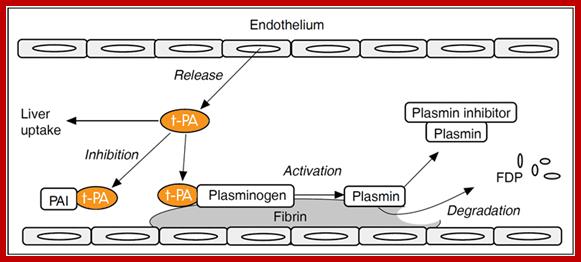
The fibrinolytic system in vivo: Plasminogen is the proenzyme of plasmin, whose primary target is the degradation of fibrin in the vasculature. The activation of plasminogen to plasmin in blood is catalyzed by t-PA secreted from endothelial cells. Fibrin provides binding sites for both plasminogen and t-PA, thereby optimizing contact between them. This mechanism ensures a high concentration of plasminogen and t-PA at the site of fibrin formation and localizes the action of plasmin. Further regulation of the system is provided by PAI-1 and plasmin inhibitor. Free t-PA, as well as complexed t-PA/PAI-1, is cleared from the circulation by receptors in the liver. Abbreviations: t-PA; tissue-type plasminogen activator, PAI-1; plasminogen activator inhibitor 1, FDP; fibrin degradation products http://diapharma.com/
ALTEOLASE (tPA) tissue plasminogen activator (Genentech 1987) used in treating Myocardial infarction; used to treat acute ischemic stroke in the following situations in which the risk of bleeding is greater than the potential benefit: current intracranial hemorrhage (ICH); subarachnoid hemorrhage; active internal bleeding; recent (within 3 months) intracranial or intraspinal surgery or serious head trauma; presence of intracranial conditions that may increase the risk of bleeding; bleeding diathesis; and current severe uncontrolled hypertension. Thrombolytic therapy for deep vein thrombosis (DVT) is to reduce the incidence of irreversible vein damage that often results when anticoagulation is given alone; It is a type of mitogen which is specific only to certain kinds of cells.
Tissue plasminogen activator (tPA, tissue-type plasminogen activator) is a serine protease found on endothelial cells (cells that line the blood vessels) involved in the breakdown of blood clots (fibrinolysis). tPA enzyme catalyzes the conversion of plasminogen to plasmin.
Effects of growth hormone on the tissues of the body can generally be described as anabolic (building up). Like most other protein hormones, GH acts by interacting with a specific receptor on the surface of cells.
Increased height during childhood is the most widely known effect of GH. Height appears to be stimulated by at least two mechanisms:
3. Because polypeptide hormones are not fat-soluble, they cannot penetrate cell membranes. Thus, GH exerts some of its effects by binding to receptors on target cells, where it activates the MAPK/ERK pathway.[35] Through this mechanism GH directly stimulates division and multiplication of chondrocytes of cartilage.
4. GH also stimulates, through the JAK-STAT signaling pathway,[35]the production of insulin-like growth factor 1 (IGF-1, formerly known as somatomedin C), a hormone homologous to proinsulin.[36] The liver is a major target organ of GH for this process and is the principal site of IGF-1 production. IGF-1 has growth-stimulating effects on a wide variety of tissues. Additional IGF-1 is generated within target tissues, making it what appears to be both an endocrine and an autocrine/paracrine hormone. IGF-1 also has stimulatory effects on osteoblast and chondrocyte activity to promote bone growth.
In addition to increasing height in children and adolescents, growth hormone has many other effects on the body:
· Increases calcium retention[citation needed], and strengthens and increases the mineralization of bone
· Increases muscle mass through sarcomere hypertrophy
· Promotes lipolysis
· Increases protein synthesis
· Stimulates the growth of all internal organs excluding the brain
· Plays a role in homeostasis
· Reduces liver uptake of glucose
· Promotes gluconeogenesis in the liver[37]
· Contributes to the maintenance and function of pancreatic islets
· Stimulates the immune system
· Increases deiodination of T4 to T3[
tPA may be manufactured using recombinant biotechnology techniques. tPA created this way may be referred to as recombinant tissue plasminogen activator (rtPA).
Human Ferritin (1010); What are our bodies made of, and how do they work? These questions are fundamental to the study of medicine and to many chemists, biologists, and bio-engineers. We know that our bodies are made up of matter as we find in soil, water and air, and thus they must be composed of atoms that have been specially arranged to produce the molecules and larger structures that sustain our lives. The life molecules are created on the surface of earth in water. These molecules have joined one to the other on chemical basis and produces Glucose sugar, amino acids, proteins, RNA and then DNA. They interacted with each other in complementary way, cohesive to each other and produced organ is substance and they joined with one another and produced life. We know that the properties of an atom (e.g., size, electronegativity, number of valence electrons) determine how that atom will interact with other atoms; furthermore, the properties and reactions of molecules depend on the properties and interactions of the atoms in the molecules. Hence, to study the human body as a complex organization of molecules that undergoes a wide array of interrelated chemical reactions, we should begin by asking one of the most basic questions about any system of molecules: What sort of atoms does the system contain? The complete answer to this question will have two main parts: 1) what elements do the atoms represent, and 2) what interactions are found between the atoms (because the properties of a given atom can be altered by interactions with other atoms).
Hence, discussion of our living primitive cells and cells of human body as a chemical system begins by answering the question, "What type of atoms does the body contain?" Of the more than 108 chemical elements known to scientists today, only a relatively small number of these elements are found in the human body. In fact, only 24 different elements are thought to be essential for the human cells and forma body. (Other elements, such as mercury, are sometimes found in the body, but do not perform any known essential or beneficial function.) The largest elemental components of the body, by mass, are oxygen (65%), carbon (18%), hydrogen (10%), and nitrogen (3%). The other elements in the body, such as calcium, phosphorus, iron, and copper, are known to physiologists as mineral elements and trace elements. Although these elements make up a much smaller percentage of the mass of the body than oxygen, carbon, hydrogen, and nitrogen, the mineral and trace elements are vital to the body's proper functioning. These elements must be present in the body in the proper amounts, and they must be available to react with other elements to form critical molecules and participate in important chemical reactions. In this tutorial, we will describe the importance of one essential trace element in the body, iron. Although, iron comprises only 0.008% of the body's mass (approximately 6 g for a 160-lb (75-kg) adult male), we cannot live without this important element in our bodies. If we concentrate all our body chemicals as a single mass it will be like the size of one sugar CUBE. http://www.chemistry.wustl.edu/
Ferritin is iron storage protein component. Iron is necessary for oxygen transport in the blood. Recall that iron is the central atom of the heme group, a metal complex that binds molecular oxygen (O2) in the lungs and carries it to all of the other cells in the body (e.g., the muscles) that need oxygen to perform their activities. In addition to hemoglobin, other important proteins in the body that contain heme groups (and therefore contain iron) include myoglobin, which takes oxygen from hemoglobin and allows the oxygen to diffuse throughout the muscle cells, and the cytochromes, which supply the body with its energy currency.
.
http://www.chemistry.wustl.edu/
This is a three-dimensional representation showing ferritin, the iron-storage protein in the body. Ferritin has a spherical shape, and iron (brown) is stored as a mineral inside the sphere.
Human Fibroblast growth factor (1020); Acidic fibroblast growth factor (FGF1) regulates a wide array of important biological phenomena such as angiogenesis, cell differentiation, tumor growth, and neurogenesis. Generally, FGFs are known for their strong affinity for the glycosamino-glycan heparin, as a prerequisite for recognition of a specific tyrosine kinase on the cell surface and are responsible for the cell signal transduction cascade. Inositol hexaphosphate (IP6) is a natural antioxidant and is known for its antiangiogenic role, in addition to its ability to control tumor growth. In the present study, we investigated the interaction of IP6 with the acidic fibroblast growth factor (FGF1) using various biophysical techniques including isothermal calorimetry, circular dichroism, and multidimensional NMR spectroscopy. Herein, we have reported the three-dimensional solution structure of the FGF1-IP6 complex. These data show that IP6 binds FGF1 and enhances its thermal stability. In addition, we also demonstrate that IP6 acts as an antagonist to acidic fibroblast growth factor by inhibiting its receptor binding and subsequently decreasing the Mitogenic activity. The inhibition likely results in the ability of IP6 to antagonize the Angiogenic and Mitogenic activity of FGF1.
Basic fibroblast growth factor is a FGF family member of the fibroblast growth factor. FGF family members possess broad mitogenic and cell survival activities, and they are involved in a variety of biological processes, including embryonic development, cell growth, morphogenesis, tissue repair, tumor growth and invasion. This protein functions as a modifier of endothelial cell migration and proliferation, as well as an Angiogenic factor. It acts as a mitogen for a variety of mesoderm- and neuroectoderm-derived cells in vitro, thus is thought to be involved in organogenesis. Three alternatively spliced variants encoding different isoforms have been described. The heparin-binding growth factors are Angiogenic agents in vivo and are potent mitogens for a variety of cell types in vitro. There are differences in the tissue distribution and concentration of these 2 growth factors. D; http://www.immunotools.de/
FGFRs belong to the family of receptor tyrosine kinases (RTK), all of which are single-pass transmembrane receptors with extracellular ligand-binding domains and an intracellular tyrosine kinase domain. Activation of RTKs by their respective ligands induces kinase activation that in turn initiates intracellular signaling networks that ultimately orchestrate key cellular processes, such as cell proliferation, growth, differentiation, migration, and survival. In this way, RTKs play pivotal biological roles during the development and adult life of multicellular organisms including cancer development.
Granulocyte Colony Stimulating Factor GM-CSF;
Amgen 1989 Neutropenia- Granulocyte Colony Stimulating Factor (G-CSF) and Granulocyte/Macrophage Colony Stimulating Factor (GM-CSF) are used widely to promote the production of granulocytes or antigen presenting cells (APC). (G-CSF (filgrastim)); Hematopoiesis is a highly proliferative (~1010 cells/day), dynamic process driven by multiple hematopoietic growth factors/cytokines (Figure). The hematopoietic growth factors are multi-functional: critical for proliferation, survival, and differentiation of hematopoietic stem, progenitor, and precursor cells to a terminally differentiated, functional cell type. Colony forming assays identified the ability of first crude supernatants, then highly purified cytokines to drive muti-lineage and single lineage differentiation. After co-culturing for 7–14 days, colonies from mononuclear cells obtained from the mouse spleen or bone marrow were measured in semisolid medium. Based on characteristics of cells within a single colony, the lineage(s) governed by the cytokine was determined. Granulocytes comprise the majority of white blood cells in human circulation and play an integral role in innate and adaptive immunity. In granulopoiesis, their production is mediated by a number of different growth factors, especially G-CSF and GM-CSF (3, 4). Due to asymmetric division some daughter cells of the hematopoietic stem cell (HSC) remain as HSC, preventing the depletion of the stem cell pool(5). Multiparameter immunophenotyping has transformed our ability to identify different cell types in hematopoiesis. Murine HSC are characterized as lin−sca1+c−kit+, and human HSC display CD34+ in the absence of lineage markers. The differentiation pathway from HSC to granulocytes is dependent on G-CSF and, less so, GM-CSF. The HSC gives rise to a common myeloid progenitor (CMP) and common lymphoid progenitor (CLP) cell(6). The CMP cells differentiate into myeloblasts, erythrocytes, and megakaryocytes via at least two intermediates, the Granulocyte/Monocyte Progenitor cell and the Erythrocyte/Megakaryocyte Progenitor cell. In the granulocytic series, myeloblasts (15–20 µm) are the first recognizable cells by their scant cytoplasm, absence of granules, and fine nucleus with nucleoli in the bone marrow clearly committed to differentiation to granulocytes. Myeloblasts differentiate into promyelocytes, which are larger (20 µm) and begin to possess granules (Figure 1B). Promyelocytes give rise to neutrophilic, basophilic, and eosinophilic precursor cells. Cell division continues through the promyelocyte stage. Fine specific granules containing inflammatory-related proteins appear during myelocyte maturation. For neutrophils, their size an nuclei become increasingly more condensed as the cells mature through myelocyte, metamyelocyte, band, and the terminally differentiated neutrophil (polymorphonuclear and ~15 µm). During episodes of stress such as infection, band cells can be found in the peripheral blood and are used as a measure of inflammation. The above process is a complex and dynamic process orchestrated by multiple cytokines and their receptors, most notably G-CSF and GM-CSF. http://mcr.aacrjournals.org/ https://www.ncbi.nlm.nih.gov; Kumar SM1, Wang HM, Mohan SK, Chou RH, Yu C.; http://www.immunotools.de/
A. The scheme of hematopoiesis from the multipotential hematopoietic stem cell to fully differentiated cell types. Principal cytokines that determine differentiation patterns in red. Epo, Erythropoietin; FLT-3 ligand, FMS-like tyrosine kinase 3 ligand; G-CSF, Granulocyte-colony stimulating factor; GM-CSF, Granulocyte Macrophage-colony stimulating factor; IL, Interleukin; M-CSF, Macrophage-colony stimulating factor; SCF, Stem Cell Factor; SDF-1, Stromal cell-derived factor-1; TGFβ, Transforming growth factor beta; TNFα, Tumour necrosis factor-alpha; Tpo, Thrombopoietin;
B. The stages of granulopoiesis from myeloblast to the mature granulocyte. During neutrophil maturation, which is driven primarily by G-CSF, granulocytic cells change shape, acquire primary and specific granules, and undergo nuclear condensation
C-CSF (Neupogen) Amgen/ 1990 Neutropenia : One of the greatest national security threats to the United States is the detonation of an improvised nuclear device or a radiological dispersal device in a heavily populated area. As such, this type of security threat is considered to be of relatively low risk, but one that would have an extraordinary high impact on health and well-being of the US citizenry. Psychological counseling and medical assessments would be necessary for all those significantly impacted by the nuclear/radiological event. Direct medical interventions would be necessary for all those individuals who had received substantial radiation exposures (e.g., >1 Gy). Although no drugs or products have yet been specifically approved by the United States Food and Drug Administration (US FDA) to treat the effects of acute radiation syndrome (ARS), granulocyte colony-stimulating factor (G-CSF), granulocyte macrophage colony-stimulating factor (GM-CSF), and pegylated G-CSF have been used off label for treating radiation accident victims. Recent threats of terrorist attacks using nuclear or radiologic devices makes it imperative that the medical community have up-to-date information and a clear understanding of treatment protocols using therapeutically effective recombinant growth factors and cytokines such as G-CSF and GM-CSF for patients exposed to injurious doses of ionizing radiation. Based on limited human studies with underlying biology, we see that the recombinants, G-CSF and GM-CSF appear to have modest, but significant medicinal value in treating radiation accident victims. In the near future, the US FDA may approve G-CSF and GM-CSF as ‘Emergency Use Authorization’ (EUA) for managing radiation-induced aplasia, an ARS-related pathology. In this article, we review the status of growth factors for the treatment of radiological/nuclear accident victims. http://www.sciencedirect.com
G-CSF and GM-CSF have been used off label for treating radiation accident victims. Radiation accident victim treatment with G-CSF and GM-CSF may have modest value.G-CSF/GM-CSF may receive Emergency Use Authorization from US FDA.
Granulocyte colony-stimulating factor; Granulocyte macrophage colony-stimulating factor; Radiation; Radiation countermeasure
Acute radiation syndrome (ARS) occurs in humans following whole-body or significant partial-body exposures to ionizing radiation with doses greater than 1 Gy, delivered at relatively high rates. Clinical components of ARS include the hematopoietic sub-syndrome (H-ARS, 2–6 Gy), gastrointestinal sub-syndrome (GIS; 6–8 Gy), and the cerebrovascular (>8 Gy) sub-syndrome [1]. However, these “sub-syndromes” tend to oversimplify the clinical reality of ARS as it often involves complex, multi-organ dysfunctions. The cerebrovascular sub-syndrome is considered incurable, whereas, individuals receiving lower radiation doses that result in either the H-ARS alone or in combination with GIS, are more likely to be amenable to countermeasures. Therefore, the latter two sub-syndromes are specific targets for the development of novel therapeutics. This is particularly the case in terms of H-ARS, that is largely driven by the radiation-induced loss of vital, growth factor-modulated hematopoietic progenitors and, in turn, by massive losses of circulating, functions blood cells, i.e., the blood cytopenias.
Colony-stimulating factors are endogenous glycoproteins that induce bone marrow hematopoietic progenitors to proliferate and differentiate into specific mature blood cell types. Granulocyte colony-stimulating factor (G-CSF) is a lineage specific colony-stimulating factor produced by monocytes, fibroblasts, and endothelial cells. It regulates the production of neutrophils within the bone marrow and affects neutrophil progenitor proliferation, differentiation, and cell activation such as enhanced phagocytic ability, respiratory burst, antibody-mediated killing , and the increased expression of cell surface antigens . G-CSF is not species-specific and several, biologically similar analogs of G-CSF have been reported (Biograstim®/Filgrastim ratiopharm/Ratiograstim®/Tevagrastim® (XM02); Zarzio® and Nivestim®). It acts by binding to a G-CSF specific transmembrane receptor (belonging to the class I cytokine receptor family), which are expressed on various hematopoietic cells such as stem cells, multi-potential progenitors, myeloid-committed progenitors, neutrophils, and monocytes. The receptor forms homo-oligomeric complexes upon ligand binding. Its mode of action and role in differentiation/maturation of cells are graphically represented in Fig. It has been approved for the following clinical indications: (a) cancer patients receiving myelosuppressive chemotherapy, (b) patients with acute myeloid leukemia receiving induction or consolidation chemotherapy, (c) cancer patients receiving bone marrow transplants (BMT), (d) patients undergoing peripheral blood progenitor cell collection and therapy, (e) patients with severe chronic neutropenia.
Binding, signal transduction and role of G-CSF in hematopoietic cell maturation/differentiation. G-CSF binds to its transmembrane receptor (G-CSFR), and initiates a signaling cascade by phosphorylating/activating Janus kinase 2 (JAK-2). The activated JAK-2 can then initiate many signaling pathways, three of which are described here in abbreviated form. Each portrayed pathway is involved in stimulating cell proliferation, cell differentiation or the inhibition of apoptosis, indicated by the pink, green and blue colored signals, respectively. Green arrows indicate stimulation and red arrows indicate inhibition. Self-replacing hematopoietic cells give rise to multi-potent stem cells, which in turn give rise to lymphoid progenitors, erythroid progenitors, megakaryocytes, basophil progenitors, eosinophil progenitors or granulocyte–monocyte progenitors. Erythroid, megakaryocyte, basophil, eosinophil progenitors give rise to erythrocytes, platelets, basophils and eosinophils, respectively. Granulocyte–monocyte progenitors give rise to neutrophils and monocytes by stimulation with G-CSF with additional cytokines and growth factors such as IL-3, GM-CSF, and M-CSF. (STAT – signal transducer and activator of transcription, STAT5 – transcription factor of 5B, STAT3 – transcription factor of 3, P indicates phosphorylated or activated signal, RAS – Rat Sarcoma, RAF – rapid accelerated fibrosarcoma – extracellular-signal-regulated kinase 5, P13K – phosphatidylinositol-4,5-bisphosphate 3-kinase (phophatidylinositide 3-kinases), BAD – Bcl-2 associated death promoter, BCLxL – B-cell lymphoma-extra-large, CASPASES – cysteine-aspartic proteases AKA cysteine-dependent aspartate-directed proteases, PDK – pyruvate dehydrogenase kinase, AKT – protein kinase B, a serine/threonine-specific protein kinase, BcL-2 – an anti-apoptotic protein, CiAP2 – Baculoviral IAP repeat-containing protein 3).
ALTEOLASE; (tPA) tissue plasminogen activator Genentech 1987 Myocardial infarction; treat acute ischemic stroke in the following situations in which the risk of bleeding is greater than the potential benefit: current intracranial hemorrhage (ICH); subarachnoid hemorrhage; active internal bleeding; recent (within 3 months) intracranial or intraspinal surgery or serious head trauma; presence of intracranial conditions that may increase the risk of bleeding; bleeding diathesis; and current severe uncontrolled hypertension. Thrombolytic therapy for deep vein thrombosis (DVT) is to reduce the incidence of irreversible vein damage that often results when anticoagulation is given alone;
It is a type of mitogen which is specific only to certain kinds of cells. Growth hormone is a 191-amino acid, single-chain polypeptide that is synthesized, stored, and secreted by somatotropic cells within the lateral wings of the anterior pituitary gland. The major isoform of the human growth hormone is a protein of 191 amino acids and a molecular weight of 22,124 daltons. The structure includes four helices necessary for functional interaction with the GH receptor.
Several molecular isoforms of GH exist in the pituitary gland and are released to blood. In particular, a variant of approximately 20 kDa originated by an alternative splicing is present in a rather constant 1:9 ratio,[9] while recently an additional variant of ~ 23-24 kDa has also been reported in post-exercise states at higher proportions. This variant has not been identified, but it has been suggested to coincide with a 22 kDa glycosylated variant of 23 kDa identified in the pituitary gland. Furthermore, these variants circulate partially bound to a protein (growth hormone-binding protein, GHBP), which is the truncated part of the growth hormone receptor, and an acid-labile subunit (ALS).
Secretion of growth hormone (GH) in the pituitary is regulated by the neurosecretory nuclei of the hypothalamus. These cells release the peptides Growth hormone-releasing hormone (GHRH or somatocrinin) and Growth hormone-inhibiting hormone (GHIH or somatostatin) into the hypophyseal portal venous blood surrounding the pituitary. GH release in the pituitary is primarily determined by the balance of these two peptides, which in turn is affected by many physiological stimulators (e.g., exercise, nutrition, sleep) and inhibitors (e.g., free fatty acids) of GH secretion
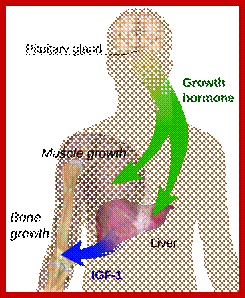
Calmodulin:
Calmodulin is a small, highly conserved protein that is 148 amino acids long (16.7 KDa). The protein has two approximately symmetrical globular domains each containing a pair of EF-hand motifs (the N- and C-domain) separated by a flexible linker region for a total of four Ca2+ binding sites;
The protein has two approximately symmetrical globular domains each containing a pair of EF-hand motifs (the N- and C-domain) separated by a flexible linker region for a total of four Ca2+ binding sites. Each EF-hand motif allows calmodulin to sense intracellular calcium levels by binding one Ca2+ ion. Calcium ion binding regions are found in the following positions in the sequence of amino acids: 21-32, 57-68, 94-105 and 130-141; each region that calcium binds to is exactly 12 amino acids long. These regions are located between two alpha helices in the EF-hand motifs, the first two regions (21-32 and 57-68) are on one side of the linker region the other two (94-105 and 130-141) are on the other side.
https://en.wikipedia.org/ Calmodulin
Calmodulin's structure is very similar to the structure of Troponin C (which is another calcium binding protein). They are both members of the EFh superfamily. Troponin C, like Calmodulin, has two globular domains that are connected by a linker region. However, Troponin C and Calmodulin differ in the length of the linker region; the linker region of Calmodulin is smaller than that of Troponin C. These remarkably similar structures are an example of how the EF hand motif is highly conserved in calcium binding proteins. Though they have similar structures, their functions are very different. Troponin C has a very specific function (to elicit a conformational change in Troponin I) ultimately causing a contraction in skeletal muscles. Calmodulin, evolved to bind a wider variety of target proteins, allowing it to play a role in many physiological events.
Calmodulin binds such a wide variety of target proteins, making it especially important for it to have flexibility. Though Calmodulin's flexibility is more evident when it is bound to a target protein, NMR studies have shown that the linker region of Calmodulin is flexible, even when it is not bound to a target protein. Another important characteristic of calmodulin that allows it to bind a large variety of target proteins is the generic shape of the non-polar grooves in the binding sites. Since the non-polar grooves are generic, they don't require the target proteins to have any specific sequence of amino acids allowing a larger variety of target proteins to be bound. Together, these two structural characteristics of calmodulin allow it to flexibly bind target proteins with various shapes and amino acid sequences. For example, calmodulin binds both NMDA receptors and potassium channels which differ in length by about 50 amino acid residues,
Calmodulin binds such a wide variety of target proteins, making it especially important for it to have flexibility. Though Calmodulin's flexibility is more evident when it is bound to a target protein, NMR studies have shown that the linker region of Calmodulin is flexible, even when it is not bound to a target protein. Another important characteristic of calmodulin that allows it to bind a large variety of target proteins is the generic shape of the non-polar grooves in the binding sites. Since the non-polar grooves are generic, they don't require the target proteins to have any specific sequence of amino acids allowing a larger variety of target proteins to be bound. Together, these two structural characteristics of calmodulin allow it to flexibly bind target proteins with various shapes and amino acid sequences. For example, calmodulin binds both NMDA receptors and potassium channels which differ in length by about 50 amino acid residues.
https://en.wikipedia.org
Role in metabolism;
Calmodulin plays an important role in the activation of phosphorylase kinase, which ultimately leads to glucose being cleaved from glycogen by glycogen phosphorylase.
Calmodulin also plays an important role in lipid metabolism by affecting Calcitonin. Calcitonin is a polypeptide hormone that lowers blood Ca2+ levels and activates G Protein cascades that leads to the generation of cAMP. The actions of calcitonin can be blocked by inhibiting the actions of calmodulin, suggesting that calmodulin plays a crucial role in the activation of calcitonin.[16]
Role in short-term and long-term memory;
Long-term potentiation (LTP) requires a depolarization of GABAergic neurons (Neurons that use GABA as a neurotransmitter) in the hippocampus. Calmodulin Kinase II (CaM-K II) plays a crucial role in achieving LTP. It contributes to the phosphorylation of an AMPA receptor which increases the sensitivity of AMPA receptors. Furthermore, research shows that inhibiting CaM-K II interferes with LTP.
Calmodulin family members; Calmodulin-like 1 (CALML1), Calmodulin 2 (CALM2), Calmodulin-like 3 (CALML3), Calmodulin-like 4 (CALML4), Calmodulin-like 5 (CALML5), Calmodulin-like 6 (CALML6).
It is a protein kinase enzyme that modifies other proteins by chemically adding phosphate groups to them (phosphorylation). Phosphorylation usually results in a functional change of the target protein (substrate) by changing enzyme activity, cellular location, or association with other proteins. The human genome contains about 500 protein kinase genes and they constitute about 2% of all human genes. Up to 30% of all human proteins may be modified by kinase activity, and kinases are known to regulate the majority of cellular pathways, especially those involved in signal transduction. Protein kinases are also found in bacteria and plants. The chemical activity of a kinase involves transferring a phosphate group from a nucleoside triphosphate (usually ATP) and covalently attaching it to specific amino acids with a free hydroxyl group. Most kinases act on both serine and threonine (serine/threonine kinases), others act on tyrosine (tyrosine kinases), and a number act on all three (dual-specificity kinases).[2] There are also protein kinases that phosphorylate other amino acids, including histidine kinases that phosphorylate histidine residues.
CAMK, also written as CaMK, is an abbreviation for the Ca2+/calmodulin-dependent protein kinase class of enzymes. CAMKs are activated by increases in the concentration of intracellular calcium ions (Ca2+) and transfers phosphates from ATP to defined serine or threonine residues in other proteins. Activated CAMK is involved in the phosphorylation of transcription factors and therefore, in the regulation of expression of responding genes. Members of this enzyme class include; CAMKI-CAMKIα, CAMKIβ, CAMKIδ’ CAMKIγ,CAMKII[3], CAMKIIα, CAMKIIβ, CAMKIIδ, CAMKIIγ,CAMKIII, CAMKIV. https://en.wikipedia.org;
Human- Factor VIII and IX (1040); Splicing of all intron-containing RNA molecules is superficially similar, as described above. However, different types of introns were identified through the examination of intron structure by DNA sequence analysis, together with genetic and biochemical analysis of RNA splicing reactions. It is produced as a zymogen, an inactive precursor. It is processed to remove the signal peptide, glycosylated and then cleaved by factor XIa (of the contact pathway) or factor VIIa (of the tissue factor pathway) to produce a two-chain form where the chains are linked by a disulfide bridge. When activated into factor IXa, in the presence of Ca2+, membrane phospholipids, and a Factor VIII cofactor, it hydrolyses one arginine-isoleucine bond in factor X to form factor Xa.
Factors VII, IX, and X all play key roles in blood coagulation and also share a common domain architecture. The factor IX protein is composed of four protein domains: the Gla domain, two tandem copies of the EGF domain and a C-terminal trypsin-like peptidase domain which carries out the catalytic cleavage. Human factor IX protein domain architecture, where each protein domain is represented by a coloured box;
![]()
https://en.wikipedia.org/wiki
Factor IX gene contains Introns: Introns were first discovered in protein-coding genes of adenovirus; Factor IX (or Christmas factor) (EC3.4.21.22) is one of the serine proteases of the coagulation system; it belongs to peptidase family S1. Deficiency of this protein causes hemophilia B. It was discovered in 1952 after a young boy named Stephen Christmas was found to be lacking this exact factor, leading to hemophilia.

Simple illustration of an unspliced mRNA precursor, with two introns and three exons (top): After the introns have been removed via splicing, the mature mRNA sequence is ready for translation (bottom). The fact that genes were split or interrupted by introns was discovered independently in 1977 by Phillip Allen Sharp and Richard J. Roberts, for which they shared the Nobel Prize in Physiology or Medicine in 1993. The term intron was introduced by American biochemist Walter Gilbert: In contrast, the mitochondrial genomes of vertebrates are entirely devoid of introns, while those of eukaryotic microorganisms may contain many introns, otherwise introns are constitutively present in most of the EUK mRNAs.
Rare diseases in Karnataka;
Gaucher’s,; Gaucher disease is a rare genetic disorder that is one of a group called lysosomal storage disorders. It is an inherited disorder that results in the accumulation of fatty molecules called cerebrosides in the body’s organs and tissues.
It is caused by a missing or deficient enzyme called ‘glucocerebrosidase‘.1 In people with Gaucher disease, the gene that would normally tell the body to produce this enzyme is altered (often called a gene mutation). The main signs and symptoms are an enlarged liver and spleen, low platelet and haemoglobin counts, and problems with bones and joints.
· There's no cure for Gaucher's disease- Replace enzymes. This approach replaces the deficient enzyme with artificial enzymes. These replacement enzymes are administered in an outpatient procedure through a vein (intravenously), typically in high doses at two-week intervals. Occasionally people experience an allergic or hypersensitivity reaction to enzyme treatment.
- Bone marrow transplantation. In this procedure, blood-forming cells that have been damaged by Gaucher's are removed and replaced, which can reverse many of Gaucher's signs and symptoms. Because this is a high-risk approach, it's performed less often than is enzyme replacement therapy.
- Spleen removal. Before enzyme replacement therapy became available, removing the spleen was a common treatment for Gaucher's disease. Currently, this procedure is typically reserved as a last resort.
- Gaucher disease (GD) is an inborn error of metabolism that affects the recycling of cellular glycolipids. It results from deficiency of a lysosomal enzyme glucocerebrosidase (also known as glucosylceramide or acid beta-glucosidase, GBA). Glucosylceramide (also called glucocerebroside) and several related compounds that ordinarily are degraded to glucose and lipid components by glucocerebrosidase accumulate within the lysosomes of cells in patients with GD.
An inherited deficiency of an enzyme (-glucosidase) which results in the buildup of a toxic substance (glucosylceramide) in different parts of the body, such as the spleen, liver, and bones.
Pompe;
Pompe disease is a rare inherited neuromuscular disorder that causes progressive muscle weakness in people of all ages. The disease is named after Johannes C. Pompe, a Dutch doctor who first described the disorder in 1932 in an infant patient. However, Pompe can affect people of all ages, with symptoms first occurring at any time from infancy to adulthood.
Pompe disease is caused by a defective gene that results in a deficiency of an enzyme, acid alpha-glucosidase (pronounced “AL-fa glue-CO-sih-days” and often abbreviated GAA). The absence of this enzyme results in excessive buildup of a substance called glycogen, a form of sugar that is stored in a specialized compartment of muscle cells throughout the body. https://www.pompe.com
The
underlying cause of Pompe disease is the same in all patients: a genetic defect
causing deficiency of an important enzyme (acid alpha-glucosidase, GAA); The
disorder is classified within several different disease categories, and doctors
may refer to it by different names The progressive nature of the disease means
that it always worsens over time, although the speed of this progression can
vary from patient to patient, read Genzymes;
Muscular dystrophy,’ http://www.medicalnewstoday.com; Muscular dystrophy is a muscle-wasting disease whose predominant forms may affect up to 1 in every 5,000 males. The condition is caused by genetic mutations that interfere with the production of muscle proteins necessary to build and maintain healthy muscles.
There are a number of muscular dystrophy types, including the following. Duchenne muscular dystrophy - the most common form of the illness. Symptoms normally start before a child's third birthday; they are generally wheelchair-bound by 12 and die of respiratory failure by their early-to-mid-twenties; Becker muscular dystrophy - similar symptoms to Duchenne but with a later onset and slower progression; death usually occurs in the mid-forties.; Myotonic (Steinert's disease) - the myotonic form is the most common adult-onset form. It is characterized by an inability to relax a muscle once it has contracted. The muscles of the face and neck are often affected first. Symptoms also include cataracts, sleepiness, and arrhythmia; Congenital - this type can be obvious from birth or before the age of 2. It affects girls and boys. Some forms progress slowly whereas others can move swiftly and cause significant impairment. Facioscapulohumeral (FSHD) - onset can be at almost any age but is most commonly seen during teenage years. The muscular weakness often begins in the face and shoulders. People with FSHD may sleep with their eyes slightly open and have trouble fully closing their eyelids. When an individual with FSHD raises their arms, their shoulder blades protrude like wings.
Limb-girdle - this variant begins in childhood or teenage years and first effects the shoulder and hip muscles. Individuals with the limb-girdle muscular dystrophy might have trouble raising the front part of the foot, making tripping a common problem.; Oculopharyngeal muscular dystrophy onset is between the ages of 40 and 70. Eyelids, throat, and face are first affected, followed by the shoulder and pelvis.
Initial symptoms; A waddling gait; Pain and stiffness in the muscles; Difficulty with running and jumping; Walking on toes; Difficulty sitting up or standing; Learning disabilities, such as developing speech later than usual; Frequent falls.
Later symptoms; Inability to walk; A shortening of muscles and tendons, further limiting movement; Breathing problems can become so severe that assisted breathing is necessary; Curvature of the spine can be caused if muscles are not strong enough to support its structure; The muscles of the heart can be weakened, leading to cardiac problems; Difficulty swallowing - this can cause aspiration pneumonia, and a feeding tube is sometimes necessary.
Muscular dystrophy is caused by mutations on the X chromosome. Each version of muscular dystrophy is due to a different set of mutations, but all prevent the body from producing dystrophin. Dystrophin is a protein essential for building and repairing muscles.
Duchenne muscular dystrophy is caused by specific mutations in the gene that encodes the cytoskeletal protein dystrophin. Dystrophin makes up just 0.002 percent of the total proteins in striated muscle, but it is an essential molecule for the general functioning of muscles.
Dystrophin is part of an incredibly complex group of proteins that allow muscles to work correctly. The protein helps anchor various components within muscle cells together and links them all to the sarcolemma - the outer membrane.
If dystrophin is absent or deformed, this process does not work correctly, and disruptions occur in the outer membrane. This weakens the muscles and can also actively damage the muscle cells themselves.
In Duchenne muscular dystrophy, dystrophin is almost totally absent; the less dystrophin that is produced, the worse the symptoms and etiology of the disease. In Becker muscular dystrophy, there is a reduction in the amount or size of the dystrophin protein.
The gene coding for dystrophin is the largest known gene in humans. More than 1,000 mutations in this gene have been identified in Duchenne and Becker muscular dystrophy.7
Diagnosing muscular dystrophy;
There are a variety of techniques used to definitively diagnose muscular dystrophy:
![[Dystrophy genetics jigsaw]](Genetic_Engineering7B-Application-Animal_Biotechnology_files/image165.jpg)
The genetic mutations involved in muscular dystrophy are well
known and can be used to make a diagnosis.
§ Enzyme assay - damaged muscles produce creatine kinase (CK). Elevated levels of CK in the absence of other types of muscle damage could suggest muscular dystrophy.
§ Genetic testing - as genetic mutations are known to occur in muscular dystrophy, these changes can be screened for.
§ Heart monitoring - electrocardiography and echocardiograms can detect changes in the musculature of the heart. This is especially useful for the diagnosis of myotonic muscular dystrophy.
§ Lung monitoring - checking lung function can give additional evidence.
§ Electromyography - a needle is placed into the muscle to measure the electrical activity. The results can show signs of muscle disease.
§ Biopsy - removing a portion of muscle and examining it under a microscope can show the tell-tale signs of muscular dystrophy.
Treatment for muscular dystrophy:
Currently, there is no cure for muscular dystrophy. Medications and various therapies help slow the progression of the disease and keep the patient mobile for the longest possible time.
Drugs
The two most commonly prescribed drugs for muscular dystrophy are:
§ Corticosteroids - although this type of medication can help increase muscle strength and slow progression, their long-term use can weaken bone and increase weight gain
§ Heart medications - if the muscular dystrophy impacts the heart, beta blockers and angiotensin-converting enzyme (ACE) inhibitors may be useful
Physical therapy
§ General exercises - a range of motion and stretching exercises can help combat the inevitable inward movement of the limbs as muscles and tendons shorten. Limbs tend to become fixed in position, and these types of activities can help keep them mobile for longer. Standard low-impact aerobic exercises such as walking and swimming can also help slow the disease's progression.
§ Breathing assistance - as the muscles used for breathing become weaker, it may be necessary to use devices to help improve oxygen delivery through the night. In the most severe cases, a patient may need to use a ventilator to breathe on their behalf.
§ Mobility aids - canes, wheelchairs, and walkers.
§ Braces - these keep muscles and tendons stretched and help slow their shortening. They also give added support to the user when moving.
Current research into muscular dystrophy
A great deal is known about the mechanisms of muscular dystrophy, both muscular and genetic, and although a full cure may be some distance away, there are avenues of research that draw ever closer to one.
Gene replacement therapy: Because the specific gene involved in muscular dystrophy has been found, a replacement gene that could create the missing dystrophin protein is a sensible consideration.
There are complicated problems with this approach, including the potential of the immune system to repel a new protein and the large size of the dystrophin gene needing to be replaced. There are also difficulties in targeting viral vectors directly to the skeletal muscle.
Another approach targets utrophin production. Utrophin is a protein similar to dystrophin that is not affected by muscular dystrophy. If utrophin production could be upregulated, the disease might be halted or slowed.
Altering protein production
If the dystrophin gene is being read by protein synthesis machinery and it reaches a mutation, it stops and does not complete the protein. Drugs are being trialed that cause the protein-making equipment to skip the mutated content and still continue to create dystrophin.
Drugs to delay muscle wasting
Rather than target the genes behind muscular dystrophy, some researchers are attempting to slow the inevitable muscle wasting.
Muscles, in standard circumstances, can repair themselves. Research into controlling or increasing these repairs could show some benefits for people with muscular dystrophy.
Stem cell research
Researchers are looking at the possibility of inserting muscle stem cells capable of producing the lacking dystrophin protein.
Current projects are looking at the most useful type of cells to use and ways in which they could be delivered to skeletal muscle.
Myoblast transplantation: During the early stages of muscular dystrophy, myoblasts (also called satellite cells) repair and replace faulty muscle fibers. As the myoblasts become exhausted, the muscles are slowly turned into connective tissue. Some studies have attempted to insert modified myoblast cells into muscles to take over from the exhausted natural myoblasts. Tim Newman
Hmophelia;
Haemophilia A is a genetic deficiency in clotting factor VIII, which causes increased bleeding and usually affects males. In the majority of cases it is inherited as an X-linked recessive trait, though there are cases which arise from spontaneous mutations Factor VII.
In terms of the symptoms of Hemophilia A there are internal or external bleeding episodes. Individuals with more severe haemophilia suffer more severe and more frequent bleeding, while others with mild haemophilia typically suffer more minor symptoms except after surgery or serious trauma. Moderate haemophiliacs have variable symptoms which manifest along a spectrum between severe and mild forms.
Prolonged bleeding from a venepuncture or heelprick is another common early sign of haemophilia, these signs may lead to blood tests which indicates haemophilia. In other people, especially those with moderate or mild haemophilia any trauma will lead to the first serious bleed.Haemophilia leads to a severely increased risk of prolonged bleeding from common injuries, or in severe cases bleeding may be spontaneous and without obvious cause. Bleeding may occur anywhere in the body, superficial bleeding such as those caused by abrasions, or shallow lacerations may be prolonged and the scab may easily be broken up due to the lack of fibrin, which may cause re-bleeding. While superficial bleeding is troublesome, some of the more serious sites of bleeding are:
Haemophilia A is inherited as an X-linked recessive trait, and occurs in males and in homozygous females (only possible in the offspring of a carrier female and a hemophilic male. However, mild haemophilia A is known to occur in heterozygous females due to X-inactivation, so it is recommended that levels of factor VIII and IX be measured in all known/potential carriers prior to surgery and in the event of clinically significant bleeding
https://en.wikipedia.org
Acquired hemophilia;
A might be caused by Ginkgo-dipyridamolum especially by Ginkgo, and it was successfully treated with hemostasis and immune-suppression therapy including methylprednisolone and cyclophosphamide. Jinbo Liu et al
We reported a case of 84-year-old women treated with Ginkgo-dipyridamolum because of thrombus of peroneal vein. She was diagnosed with acquired hemophilia A for high-titer factor VIII (FVIII) inhibitor and lower level of FVIII activity. aPTT returned to normal range after treated with corticosteroids and cyclophosphamide. Acquired hemophilia A (AHA) is a bleeding disorder caused by an autoantibody to factor VIII (FVIII), presenting with serious and fatal bleeding such as subcutaneous and gastrointestinal bleeding [1]. Therapy of bleeding control and immune suppression must be given immediately to save patient’s life [2]. Patients with bleeding and prolonged level of activated partial thromboplastin time (aPTT) with normal level of bleeding time, prothrombin time, and thrombin time should be considered as this disease. Evidence of higher level of FVIII inhibitor titer and lower level of FVIII activity will confirm the diagnosis of this disease [3]. Ginkgo-dipyridamolum is used for the treatment of venous thromboembolism approved by China Food and Drug Administration. Here, we reported a rare case of AHA might be related with Ginkgo-dipyridamolum, and it was successfully treated with hemostasis and immune-suppression therapy, http://onlinelibrary.wiley.com/doi
Thalassemia;
Thalassemia 2016: Modern medicine battles an ancient disease Deborah Rund*. Thalassemia was first clinically described nearly a century ago and treatment of this widespread genetic disease has greatly advanced during this period. DNA-based diagnosis elucidated the molecular basis of the disease and clarified the variable clinical picture. It also paved the way for modern methods of carrier identification and prevention via DNA-based prenatal diagnosis. Every aspect of supportive care, including safer blood supply, more regular transfusions, specific monitoring of iron overload, parenteral and oral chelation, and other therapies, has prolonged life and improved the quality of life of these patients. Significant advances have also been made in allogenic bone marrow transplantation, the only curative therapy. Recently, there has been a rejuvenated interest in studying thalassemia at the basic science level, leading to the discovery of previously unknown mechanisms leading to anemia and enabling the development of novel therapies. These will potentially improve the treatment of, and possibly cure the disease. Pathways involving activin receptors, heat shock proteins, JAK2 inhibitors and macrophage targeted therapy, among others, are being studied or are currently in clinical trials for treating thalassemia. Novel types of genetic therapies are in use or under investigation. In addition to the challenges of treating each individual patient, the longer survival of thalassemia patients has raised considerations regarding worldwide control of thalassemia, since prevention is not universally implemented. This review will trace a number of the original medical milestones of thalassemia diagnosis and treatment, as well as some of the most recent developments which may lead to innovative therapeutic modalities. Am. J. Hematol. 91:15–21, 2016. VC 2015 Wiley Periodicals, Inc.
MPS-mucopolysaccharidosis;
Mucopolysaccharidoses (MPS) and related diseases are genetic lysosomal storage diseases (LSD) caused by the body’s inability to produce specific enzymes. Normally, the body uses enzymes to break down and recycle materials in cells. In individuals with MPS and related diseases, the missing or insufficient enzyme prevents the proper recycling process, resulting in the storage of materials in virtually every cell of the body. As a result, cells do not perform properly and may cause progressive damage throughout the body, including the heart, bones, joints, respiratory system and central nervous system. While the disease may not be apparent at birth, signs and symptoms develop with age as more cells become damaged by the accumulation of cell materials. http://mpssociety.org
Characteristics of MPS and Related Diseases;?
While the symptoms of the diseases may vary from one syndrome to another, there are similarities. Affected individuals may have mental retardation, cloudy corneas, short stature, stiff joints, incontinence, speech and hearing impairment, chronic runny nose, hernia, heart disease, hyperactivity, depression, pain and a dramatically shortened life span.
How Are These Diseases Inherited?
MPS and related diseases are hereditary. In nearly all cases a child receives a recessive gene from each parent. MPS II is the only exception where the gene may be passed from a mother to her male children. A couple’s chance of having another child with one of these diseases is 1 in 4 with each pregnancy. Unaffected siblings may be gene carriers of the disease. The occurrence of MPS and related diseases in the general population is thought to be one in 25,000 births.
Is Prenatal Diagnosis or Carrier Detection Testing Available?
For most MPS conditions, amniocentesis can be performed between 14 and 17 weeks gestation to determine if the unborn child is affected. Alternatively, chorionic villus sampling (CVS) can be performed between eight and ten weeks of pregnancy. Tests also are available to determine whether individuals are carriers of an MPS or related disease. To learn more about these tests contact your doctor, nearest genetic center or the National MPS Society.
Is Research Helping Today’s Families?
Although there is currently no cure for MPS or related diseases, research is making great strides. Carrier detection, the development of replacement enzymes, and the possibility of gene therapy, are among today’s research themes and treatment options. Bone marrow transplantation has been considered successful for many, though relatively few individuals qualify for this high-risk procedure. We’ve made major advancements in research thanks to the fundraising efforts of the Society and its members.
How Can You Help Find the Cure?
Since 1975 the Society has supported individuals and families affected with MPS and related diseases. We are governed by a member-elected volunteer Board of Directors, many of whom are parents of children with MPS and related diseases. We benefit from the expertise of a Scientific Advisory Board, comprised of world-class physicians, researchers and medical professionals throughout the world. We need your support in helping us teach our mission to others and raising the money we need to support medical research – the key to longer, happier lives with MPS.
Autism spectrum disorder;
Definition; Autism spectrum disorder (ASD) is the name for a group of developmental disorders. ASD includes a wide range, “a spectrum,” of symptoms, skills, and levels of disability.
People with ASD often have these characteristics:
Ongoing social problems that include difficulty communicating and interacting with others; -Repetitive behaviors as well as limited interests or activities; -Symptoms that typically are recognized in the first two years of life; -Symptoms that hurt the individual’s ability to function socially, at school or work, or other areas of life.
Some people are mildly impaired by their symptoms, while others are severely disabled. Treatments and services can improve a person’s symptoms and ability to function. Families with concerns should talk to their pediatrician about what they’ve observed and the possibility of ASD screening. According to the Centers for Disease Control and Prevention (CDC) around 1 in 68 children has been identified with some form of ASD.
https://www.nimh.nih.gov/in; What is the difference between Asperger’s syndrome and Autism spectrum disorder ASD?
In the past, Asperger’s syndrome and Autistic Disorder were separate disorders. They were listed as subcategories within the diagnosis of “Pervasive Developmental Disorders.” However, this separation has changed. The latest edition of the manual from the American Psychiatric Association, the Diagnostic and Statistical Manual of Mental Disorders (DSM-5), does not highlight subcategories of a larger disorder. The manual includes the range of characteristics and severity within one category. People whose symptoms were previously diagnosed as Asperger’s syndrome or Autistic Disorder are now included as part of the category called Autism Spectrum Disorder (ASD).
Restrictive / repetitive behaviors may include:
Repeating certain behaviors or having unusual behaviors; Having overly focused interests, such as with moving objects or parts of objects; Having a lasting, intense interest in certain topics, such as numbers, details, or facts.
Social communication / interaction behaviors may include:
Getting upset by a slight change in a routine or being placed in a new or overly stimulating setting; Making little or inconsistent eye contact; Having a tendency to look at and listen to other people less often; Rarely sharing enjoyment of objects or activities by pointing or showing things to others; Responding in an unusual way when others show anger, distress, or affection; Failing to, or being slow to, respond to someone calling their name or other verbal attempts to gain attention; Having difficulties with the back and forth of conversations; Often talking at length about a favorite subject without noticing that others are not interested or without giving others a chance to respond; Repeating words or phrases that they hear, a behavior called echolalia; Using words that seem odd, out of place, or have a special meaning known only to those familiar with that person’s way of communicating; Having facial expressions, movements, and gestures that do not match what is being said; Having an unusual tone of voice that may sound sing-song or flat and robot-like; Having trouble understanding another person’s point of view or being unable to predict or understand other people’s actions.; People with ASD may have other difficulties, such as being very sensitive to light, noise, clothing, or temperature. They may also experience sleep problems, digestion problems, and irritability.
ASD is unique in that it is common for people with ASD to have many strengths and abilities in addition to challenges.
Strengths and abilities may include: Having above-average intelligence – the CDC reports 46% of ASD children have above average intelligence.
· Being able to learn things in detail and remember information for long periods of time
· Being strong visual and auditory learners
· Exceling in math, science, music, or art.
Diagnosing ASD;
Doctors diagnose ASD by looking at a child’s behavior and development. Young children with ASD can usually be reliably diagnosed by age two. Older children and adolescents should be evaluated for ASD when a parent or teacher raises concerns based on watching the child socialize, communicate, and play.
Diagnosing ASD in adults is not easy. In adults, some ASD symptoms can overlap with symptoms of other mental health disorders, such as schizophrenia or attention deficit hyperactivity disorder (ADHD). However, getting a correct diagnosis of ASD as an adult can help a person understand past difficulties, identify his or her strengths, and obtain the right kind of help.
Diagnosis in young children is often a two-stage process:
Stage 1: General Developmental Screening During Well-Child Checkups
Every child should receive well-child check-ups with a pediatrician or an early childhood health care provider. The Centers for Disease Control and Prevention (CDC) recommends specific ASD screening be done at the 18- and 24-month visits.
Earlier screening might be needed if a child is at high risk for ASD or developmental problems. Those at high risk include children who:
· Have a sister, brother, or other family member with ASD
· Have some ASD behaviors
· Were born premature, or early, and at a low birth weight.
Parents’ experiences and concerns are very important in the screening process for young children. Sometimes the doctor will ask parents questions about the child’s behaviors and combine this information with his or her observations of the child. Read more about screening instruments on the CDC website.
Children who show some developmental problems during this screening process will be referred for another stage of evaluation.
Stage 2: Additional Evaluation
This evaluation is with a team of doctors and other health professionals with a wide range of specialties who are experienced in diagnosing ASD. This team may include:
· A developmental pediatrician—a doctor who has special training in child development
· A child psychologist and/or child psychiatrist—a doctor who knows about brain development and behavior
· A speech-language pathologist—a health professional who has special training in communication difficulties.
The evaluation may assess:
· Cognitive level or thinking skills
· Language abilities
· Age-appropriate skills needed to complete daily activities independently, such as eating, dressing, and toileting.
Because ASD is a complex disorder that sometimes occurs along with other illnesses or learning disorders, the comprehensive evaluation may include:
· Blood tests
· Hearing test
The outcome of the evaluation will result in recommendations to help plan for treatment.
Diagnosis in older children and adolescents
Older children whose ASD symptoms are noticed after starting school are often first recognized and evaluated by the school’s special education team. The school’s team may refer these children to a health care professional.
Parents may talk with a pediatrician about their child’s social difficulties including problems with subtle communication. These subtle communication issues may include understanding tone of voice, facial expressions, or body language. Older children may have trouble understanding figures of speech, humor, or sarcasm. Parents may also find that their child has trouble forming friendships with peers. The pediatrician can refer the child for further evaluation and treatment.
Diagnosis in adults
Adults who notice the signs and symptoms of ASD should talk with a doctor and ask for a referral for an ASD evaluation. While testing for ASD in adults is still being refined, adults can be referred to a psychologist or psychiatrist with ASD expertise. The expert will ask about concerns, such as social interaction and communication challenges, sensory issues, repetitive behaviors, and restricted interests. Information about the adult’s developmental history will help in making an accurate diagnosis, so an ASD evaluation may include talking with parents or other family members.
Retinoblastoma ; Retinoblastoma is a pediatric cancer that requires a careful integration of multidisciplinary care. Treatment of retinoblastoma aims to save the patient's life and preserve useful vision and, therefore, needs to be individualized. The management of intraocular retinoblastoma has evolved to a more risk-adapted approach that aims to minimize systemic exposure to drugs, optimize ocular drug delivery, and preserve useful vision. For patients presenting with extraocular retinoblastoma, treatment with intensive chemotherapy is required, including consolidation with high-dose chemotherapy and autologous hematopoietic stem cell rescue. While most patients with orbital disease and a large proportion of patients with systemic extra–central nervous system (CNS) metastases can be cured, the prognosis for patients with intracranial disease is dismal. Retinoblastoma arises from the retina, and its growth is usually under the retina and toward the vitreous. Involvement of the ocular coats and optic nerve occurs as a sequence of events as the tumor progresses. Invasion of the choroid is common, although occurrence of massive invasion is usually limited to advanced disease. After invading the choroid, the tumor gains access to systemic circulation and creates the potential for metastases. Further progression through the ocular coats leads to invasion of the sclera and the orbit. Anteriorly, tumor invading the anterior chamber may gain access to systemic circulation through the canal of Schlemm. Progression through the optic nerve and past the lamina cribrosa increases the risk of systemic and CNS dissemination. https://www.cancer.gov/
Causes of Retinoblastoma-Related Mortality
While retinoblastoma is a highly curable disease, the challenge for those who treat retinoblastoma is to preserve life and to prevent the loss of an eye, blindness, and other serious effects of treatment that reduce the patient's life span or quality of life. With improvements in the diagnosis and management of retinoblastoma over the past several decades, metastatic retinoblastoma is observed less frequently in the United States and other developed nations. As a result, other causes, such as trilateral retinoblastoma and subsequent neoplasms (SNs), have become significant contributors to retinoblastoma-related mortality in the first and subsequent decades of life.
Death from a second neoplasm is the most common cause of death and contributes to more than 50% of deaths in patients with bilateral disease. In the United States, before the advent of chemoreduction as a means of treating heritable or bilateral disease and the implementation of neuroimaging screening, trilateral retinoblastoma contributed to more than 50% of retinoblastoma-related mortality in the first decade after diagnosis.
Trilateral retinoblastoma
Trilateral retinoblastoma is a well-recognized syndrome that occurs in 5% to 15% of patients with heritable retinoblastoma. It is defined by the development of an intracranial midline neuroblastic tumor, which typically develops between the ages of 20 and 36 months.
Trilateral retinoblastoma has been the principal cause of death from retinoblastoma in the United States during the first decade of life. Because of the poor prognosis for patients with trilateral retinoblastoma and the apparent improved survival with early detection and aggressive treatment, screening with routine neuroimaging could potentially detect most cases within 2 years of first diagnosis. Routine baseline brain MRI is recommended at diagnosis because it may detect trilateral retinoblastoma at a subclinical stage. In a small series of patients, the 5-year overall survival rate was 67% for those detected at baseline, compared with 11% for the group with a delayed diagnosis. Although it is not clear whether early diagnosis can impact survival, screening with MRI has been recommended as often as every 6 months for 5 years for patients suspected of having heritable disease or those with unilateral disease and a positive family history. Computed tomography scans are generally avoided for routine screening in these children because of the risk related to ionizing radiation exposure.
A cystic pineal gland, which is commonly detected by surveillance MRI, needs to be distinguished from a cystic variant of pineoblastoma. In children without retinoblastoma, the incidence of pineal cysts has been reported to be 55.8% In a case-control study that included 77 children with retinoblastoma and 77 controls, the incidence of pineal cysts was similar (61% and 69%, respectively), and the size and volume of the pineal gland was not significantly different between the groups. However, a cystic component has been described in up to 57% of patients with histologically confirmed trilateral retinoblastoma. An excessive increase in the size of the pineal gland seems to be the strongest parameter indicating a malignant process.
Subsequent neoplasms (SNs);
Survivors of retinoblastoma have a high risk of developing SNs. Factors that influence this risk include the following:
Heritable retinoblastoma. Patients with heritable retinoblastoma have a markedly increased incidence of SNs, independent of treatment with radiation therapy. A possible association between the type of RB1 mutation and incidence of SNs may exist, with complete loss of RB1 activity associated with a higher incidence of SNs. With the increase in survival of patients with heritable retinoblastoma, it has become apparent that they are also at risk of developing epithelial cancers late in adulthood. A marked increase in mortality from lung, bladder, and other epithelial cancers has been described. Among retinoblastoma survivors with heritable retinoblastoma, those with an inherited germline mutation are at a slightly higher risk of developing an SN than are those with a de novo mutation; this increase appears to be most significant for melanoma.
Anatomy of the eye showing the outside and inside of the eye, including the eyelid, pupil, sclera, iris, ciliary body, canal of Schlemm, cornea, lens, vitreous humor, retina, choroid, optic nerve, and lamina cribrosa. The vitreous humor is a gel that fills the center of the eye.
Past treatment for retinoblastoma with radiation therapy.
The cumulative incidence of SNs was reported to be 26% (± 10%) in nonirradiated patients and 58% (± 10%) in irradiated patients by 50 years after diagnosis of retinoblastoma—a rate of about 1% per year. ] A German series of 633 patients with heritable retinoblastoma demonstrated a 5-year survival of 93%; however, 40 years later, only 80% of patients survived, with most succumbing to radiation-induced SNs (hazard ratio, approximately 3).] Other studies analyzing cohorts of patients treated with more advanced radiation planning and delivery technology have reported the SN rates to be about 9.4% in nonirradiated patients and about 30.4% in irradiated patients. In a nonrandomized study that compared two contemporary cohorts of patients with hereditary retinoblastoma who were treated with either photon (n = 31) or proton (n = 55) therapy, the 10-year cumulative incidence of radiation-induced SNs was significantly different between the two groups (0% for proton radiation vs. 14% for photon radiation, P = .015). A longer follow-up will be required to further define the risk of SNs associated with proton radiation.
The most common SN is sarcoma, specifically osteosarcoma, followed by soft tissue sarcoma and melanoma; these malignancies may occur inside or outside of the radiation field, although most are radiation induced. The carcinogenic effect of radiation therapy is associated with the dose delivered, particularly for subsequent sarcomas; a step-wise increase is apparent at all dose categories. In irradiated patients, two-thirds of SNs occur within irradiated tissue, and one-third of SNs occur outside the radiation field.
Retinitis pigmentosa:
Retinitis pigmentosa (RP) is an inherited, degenerative eye disease that causes severe vision impairment due to the progressive degeneration of the rod photoreceptor cells in the retina. This form of retinal dystrophy manifests initial symptoms independent of age; thus, RP diagnosis occurs anywhere from early infancy to late adulthood. Patients in the early stages of RP first notice compromised peripheral and dim light vision due to the decline of the rod photoreceptors. The progressive rod degeneration is later followed by abnormalities in the adjacent retinal pigment epithelium (RPE) and the deterioration of cone photoreceptor cells. As peripheral vision becomes increasingly compromised, patients experience progressive "tunnel vision" and eventual blindness. Affected individuals may additionally experience defective light-dark adaptations, nyctalopia (night blindness), and the accumulation of bone spicules in the fundus. https://en.wikipedia.org
Author: Victoria White
The first patient has been treated with ReNeuron’s cell therapy candidate for the blindness-causing disease retinitis pigmentosa in a first-in-human US clinical trial. 15 March 2016 • Author: Victoria White

https://www.europeanpharmaceuticalreview.com
FragileX; Fragile X syndrome (FXS) is the most common inherited form of intellectual disability, and is the leading single-gene cause of autism spectrum disorders. It is due to a loss of the fragile X mental retardation protein, which leads to molecular, behavioral, and cognitive deficits in these patients. Improvements in our understanding of its pathophysiology have led to the development of numerous targeted treatments in FXS as highlighted by metabotropic glutamate receptor antagonists and gamma-Aminobutyric acid receptor modulators. This review will summarize relevant pre-clinical data and results from clinical trials in human subjects with FXS. Spinal Muclular atrophy SMA; Spinal muscular atrophy (SMA), also called autosomal recessive proximal spinal muscular atrophy in order to distinguish it from other conditions with similar name, is a rare neuromuscular disorder characterised by loss of motor neurons and progressive muscle wasting, often leading to early death. https://en.wikipedia.org https://www.ncbi.nlm.nih.gov/ Fragile X syndrome (FXS) is the most common cause of inherited intellectual disability with prevalence rates estimated at 1:5,000 males and 1:8,000 females (1). Its etiology is due to a cytosine-guanine-guanine (CGG) repeat expansion mutation in the FMR1 gene located on the long arm of the X chromosome. The normal range for individuals is up to 44 CGG repeats, whereas patients with FXS have >200. Above this threshold, the gene becomes methylated and silenced, resulting in significantly reduced or absent levels of the FMR1 gene product (FMRP). FMRP is a RNA-binding protein that is heavily expressed in neurons (2,3), and functions in the stability, localization, and translation of select mRNAs
The disorder is caused by a genetic defect in the SMN1 gene, which encodes SMN, a protein widely expressed in all eukaryotic cells and necessary for survival of motor neurons. Lower levels of the protein results in loss of function of neuronal cells in the anterior horn of the spinal cord and subsequent system-wide muscle wasting (atrophy). Spinal muscular atrophy is an inherited disorder and is passed on in an autosomal recessive manner.
In December 2016, nusinersen became the first approved drug to treat SMA while several other compounds remain in clinical trials.
Approximately one out of every 40 individuals in the United States is a carrier of the gene responsible for spinal muscular atrophy, a neurodegenerative disease that causes muscles to weaken. Researchers have developed a new molecule in April 2014 that was found to be highly effective in animal models. Now, testing of that compound is leading to a better prognosis for mice with the disease and the possibility of potential drugs.
"It's a type of molecule called an antisense oligonucleotide, or ASO, that essentially is synthetic string of nucleic acid that binds a specific sequence in the gene." Fortunately, humans have a nearly identical copy gene called SMN2, however, SMN2 normally only makes a small amount of the correct SMN protein. Lorson's compound targets SMN2 and effectively "turns the volume up" for SMN2, allowing it to make more of the correct SMN protein.
"Our current treatment helps the body create a backup mechanism to combat the disease and extends survival in mice with SMA from just 13 days to a little over five months after only one injection at birth," Lorson said. "This treatment helps produce the right form of SMN, the one that was only produced at very low levels before." Lorson
The study, "Optimization of Morpholino Antisense Oligonucleotides Targeting the Intronic Repressor Element1 in Spinal Muscular Atrophy," recently was accepted for publication in Molecular Therapy, a journal of Nature. Previous funding was received from CureSMA. Erkan Osman, a postdoctoral fellow and lead author on this publication working in Lorson's lab is funded by FightSMA and the Gwendolyn Strong Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies. bu.edu
Alzheimer disease; Pharmaceuticals investigating or developing 77 medicines to help the more than 5 million patients in the United States . United States will increase to 13.8 million, and cost of care will increase to $1.1 trillion by 2050, according to the Alzheimer’s Association. http://phrma-docs.phrma.org Plaques are abnormal clusters of beta-amyloid protein fragments between nerve cells, while tangles are twisted fibers made primarily of a protein called “tau” that accumulates in brain cells, damaging and killing them. Researchers are also looking at the role inflammation and insulin resistance play in Alzheimer’s disease. AC-1204 Accera mild to moderate Alzheimer's disease Phase III (glucose stimulant) Broomfield, CO. www.accerapharma.com, biogen
Drugs: Huperzine Primary immune difficiencyPID:
Marfan syndrome:; Marfan syndrome is caused by mutations in the fibrillin-1 gene (FBN1). The most important features affect the cardiovascular system, eyes, and skeleton. Marfan syndrome is caused by mutations in the fibrillin-1 gene (FBN1). Marfan syndrome (MFS) is a genetic disorder of the connective tissue. https://en.wikipedia.org
Marfan syndrome is a genetic disorder that affects the body’s connective tissue.
Connective tissue is made up of proteins. The protein that plays a role in Marfan syndrome is called fibrillin-1. Marfan syndrome is caused by a defect (or mutation) in the gene that tells the body how to make fibrillin-1. This mutation results in an increase in a protein called transforming growth factor beta, or TGF-β. The increase in TGF-β causes problems in connective tissues throughout the body, which in turn creates the features and medical problems associated with Marfan syndrome and some related disorders.
People with Marfan syndrome are born with it, but features of the disorder are not always present right away. Some people have a lot of Marfan features at birth or as young children – including serious conditions like aortic enlargement. Others have fewer features when they are young and don’t develop aortic enlargement or other signs of Marfan syndrome until they are adults. Some features of Marfan syndrome, like those affecting the heart and blood vessels, bones or joints, can get worse over time. This makes it very important for people with Marfan syndrome and related disorders to receive accurate, early diagnosis and treatment. Without it, they can be at risk for potentially life-threatening complications. The earlier some treatments are started, the better the outcomes are likely to be.
Knowing the signs of Marfan syndrome can save lives. Our community of experts estimates that nearly half the people who have Marfan syndrome don’t know it. This is something we are working hard to change.

https://www.marfan.org
Every person’s experience with Marfan syndrome is slightly different. No one has every feature and people have different combinations of features. Some features of Marfan syndrome are easier to see than others. These include:
· Long arms, legs and fingers, Tall and thin body type, Curved spine, Chest sinks in or sticks out, Flexible joints
· Flat feet, Crowded teeth, Stretch marks on the skin that are not related to weight gain or loss.
·
· Marfan syndrome is caused by a change in the gene that controls how the body makes fibrillin, an essential component of connective tissue that contributes to its strength and elasticity.
People with Marfan syndrome may have: A tall, thin build. Long arms, legs, fingers, and toes and flexible joints. Scoliosis, or curvature of the spine. A chest that sinks in or sticks out. Crowded teeth. Flat feet.
· Medications are typically not used to treat Marfan syndrome. However, your doctor may prescribe a beta-blocker, which decreases the forcefulness of the heartbeat and the pressure within the arteries, thus preventing or slowing the enlargement of the aorta. Beta-blocker therapy is usually started when the person with Marfan syndrome is young.
· Some people are unable to take beta-blockers because they have asthma or because of the medication's side effects, which may include drowsiness or weakness, headaches, slowed heartbeat, swelling of the hands and feet or trouble breathing and sleeping. In these cases, another medication called a calcium channel blocker may be recommended.
· An ongoing clinical trial that began in 2007 is looking at the how two drugs, atenolol, a beta-blocker that may slow the growth of the aorta, and losartan, an angiotension receptor blocker used to lower blood pressure, can be used to manage Marfan syndrome
· Surgery; The goal of surgery for Marfan syndrome is to prevent aortic dissection or rupture and to treat problems affecting the heart's valves, which control the flow of blood in and out of the heart and between the heart's chambers. http://www.webmd.com
Myasthenis gravis; http://www.healthline.com/
Myasthenia gravis (MG) is a neuromuscular disorder that causes weakness in the skeletal muscles, which are the muscles your body uses for movement. It occurs when communication between nerve cells and muscles becomes impaired. This impairment prevents crucial muscle contractions from occurring, resulting in muscle weakness. According to the Myasthenia Gravis Foundation of America, MG is the most common primary disorder of neuromuscular transmission. It’s a relatively rare condition that affects between 14 and 20 out of every 100,000 people in the United States.
The main symptom of MG is weakness in the voluntary skeletal muscles, which are muscles under your control. The failure of muscles to contract normally occurs because they can’t respond to nerve impulses. Without proper transmission of the impulse, a blocked communication occurs between nerve and muscle and weakness results. Weakness associated with MG typically gets worse with more activity and improves with rest. Symptoms of MG can include:
· Trouble talking; problems, walking up stairs or lifting objects’ facial paralysis; difficulty breathing because of muscle weakness; difficulty swallowing or chewing; fatigue; hoarse voice; drooping of eyelids; double vision.
Symptoms of MG can include: MG is a neuromuscular disorder that’s usually caused by an autoimmune problem. Autoimmune disorders occur when your immune system mistakenly attacks healthy tissue. In this condition, antibodies, which are proteins that normally attack foreign, harmful substances in the body, attack the neurotransmitter substance acetylcholine, which is a crucial substance for communication between nerve cells and muscles. This results in muscle weakness.
Medication
Corticosteroids and immunosuppressants can be used to suppress the immune system. These medications help minimize the abnormal immune response that occurs in MG. Additionally, cholinesterase inhibitors, such as pyridostigmine (Mestinon), can be used to increase communication between nerves and muscles.
Plasmapheresis is also known as a plasma exchange. This process removes harmful antibodies from the blood, which may result in an improvement in muscle strength. Plasmapheresis is a short-term treatment. The body continues to produce the harmful antibodies and weakness may recur. Plasma exchange is helpful before surgery or during times of extreme MG weakness.
Intravenous Immune Globulin
Intravenous immune globulin (IVIG) is blood product that comes from donors. It’s used to treat autoimmune MG. Although it’s not entirely known how IVIG works, it affects the creation and function of antibodies.
Parkinson disease; The array of pharmacologic and surgical treatments available for the treatment of idiopathic (or Lewy body) Parkinson disease (PD) is broader than for any other degenerative disease of the central nervous system. Management of individual patients requires careful consideration of a number of factors, including the patient's symptoms and signs, age, stage of disease, degree of functional disability, and level of physical activity and productivity. Treatment can be divided into pharmacologic, nonpharmacologic, and surgical therapy. http://www.uptodate.com
The pharmacologic treatment of PD can be further divided into neuroprotective and symptomatic therapy. In practice, nearly all of the available treatments are symptomatic in nature and do not appear to slow or reverse the natural course of the disease. However, several potential neuroprotective agents for PD have shown some promise in animals and/or humans and are undergoing further investigation. Neuroprotective therapy for PD is discussed in greater detail separately. (See "Neuroprotective therapy for Parkinson disease".)
Approach Considerations
Levodopa, coupled with carbidopa, a peripheral decarboxylase inhibitor (PDI), remains the gold standard of symptomatic treatment for Parkinson disease. Carbidopa inhibits the decarboxylation of levodopa to dopamine in the systemic circulation, allowing for greater levodopa distribution into the central nervous system. Levodopa provides the greatest antiparkinsonian benefit for motor signs and symptoms, with the fewest adverse effects in the short term; however, its long-term use is associated with the development of motor fluctuations (“wearing-off”) and dyskinesias. Once fluctuations and dyskinesias become problematic, they are difficult to resolve.
Dopamine agonists (ropinirole, pramipexole) provide moderate symptomatic benefit and delay the development of dyskinesia compared with levodopa. Proactively screen patients receiving oral dopamine agonists for adverse events. A review of the Cochrane and PubMed databases from 1990 to 2008 found that these agents caused a 15% increase in adverse events such as somnolence, sudden-onset sleep, hallucinations, edema, and impulse control disorders (eg, pathologic gambling, shopping, and Internet use; hypersexuality; and hoarding). Note that patients may be reluctant to mention these events or may not attribute them to their treatment. http://emedicine.medscape.com
Pre-Symptomatic;
· Stages 1-2 (Shultz-Shaeffer, 2010; Sveinbjornsdottir, 2016; Wu & Frucht, 2005): Abnormal clumps of a protein structure called alpha-synuclein (Lewy bodies) disrupt the normal functioning of nerve cells. In the first stages of PD, Lewy bodies form in the medulla oblongata, pontine tegmentum, olfactory bulb, and anterior olfactory nucleus.
· Along with this, genetic and environmental influences can increase the probability of PD development.
Clinical symptoms
· Stages 3-4 (Jankovic, 2008; Sveinbjornsdottir, 2016): Lewy bodies start to form in the forebrain and midbrain areas. Degeneration and Lewy bodies start to appear in the dopamine nerve cells of the basal ganglia and substantia nigra. Degeneration of the dopaminergic nigrostriatal neurons constitute the primary feature of motor dysfunction in PD. Symptoms include: tremor, rigidity, bradykinesia and postural instability.
· Note: Research suggests (e.g., Sveinbjornsdottir, 2016) that clinical symptoms manifest at this stage, although evidence is often hard to verify. This is often because individuals with PD do not present for clinical assessment until degeneration has impaired their standard of living; stages 5 and 6.
· Stages 5-6 (Jankovic, 2008; Sveinbjornsdottir, 2016): At this stage abnormalities become apparent in the neocortex, which is involved in higher order functioning such as sensory perception, motor commands, spatial reasoning and language. Neurodegeneration may also be apparent in other neural pathways, giving rise to previously mentioned neuropsychiatric symptoms.
- At this stage both motor and non-motor symptoms are present. https://en.wikiversity.org
The role of dopamine in PD;
Dopamine plays an integral role in the physiological motivation of controlled motor movement in the body (Jankovic, 2008). Along this line, the loss of dopamine facilitating neurons in the substantia nigra, disturbs normal neurological functioning, changing a person's physical control over motor movements (Jankovic, 2008). As neurons start to degenerate in the substantia nigra, its role as a facilitator for movement readiness also starts to diminish (Kalat, 2014). The loss of dopamine-releasing axons means there is also a loss of axons to the ventral striatum, which is strongly associated with reward, risk and decision making (Kalat, 2014). As a consequence of losing these neural connections, the ventral striatum, decreases its inhibition of the globus pallidus, which is responsible for the regulation of voluntary movement, working closely with the cerebellum to create smooth controlled movement (Kalat, 2014). In turn, the globus pallidus inhibits the thalamus which communicates sensory motor information between the sub-cortical areas and the cortex (Kalat, 2014).
The end result? People with PD are still capable of controlled movement, but a communication breakdown in dopamine facilitating pathways, causes involuntary movement problems such as tremor, poor balance and coordination (NINDS, 2016). It can also result in voluntary movement issues such as "freezing" (hypokinesia), and gait problems such as slowness of movement (Sveinbjornsdottir, 2016). https://en.wikiversity.org
Levodopa (L-dopa)
In the management of PD, L-dopa, the chemical precursor to the neurotransmitter dopamine, is the gold standard for management of PD (NINDS, 2016). The biggest reason L-dopa has been so effective, has been its ability to bypass the blood brain barrier, replenishing the brains dwindling supply of dopamine (Cools, 2006; Hornykiewicz, 2002). The replenishment of dopamine in areas such as the substantia nigra, means that there is more dopamine available to facilitate movement (NINDS, 2016). As a result, L-dopa primarily increases daily function by reducing the severity of symptoms such as bradykinesia, rigidity and, in some cases, tremor (Fahn, 2008, NINDS, 2016). Concurrently, carbidopa and other agonists are used in conjunction with L-dopa, delaying the metabolism of dopamine, and thereby increasing the availability of dopamine in degenerated dopaminergic nerve cells (Fahn, 2008; NINDS, 2016).
Treatments for PD have progressed in small steps, with a fairly stable theory of why PD occurs, and treatments that are evolving but which are not truly understood (Jankovic, 2008). Deep Brain Stimulation (DBS) is used as the main treatment for severe cases of PD.
Osteoporosis: Weakening of bone due to bone marrow density decreases. Key risk factors for osteoporosis include genetics, lack of exercise, lack of calcium and vitamin D, personal history of fracture as an adult, cigarette smoking, excessive alcohol consumption, history of rheumatoid arthritis, low body weight, and family history of osteoporosis. The diagnosis of osteoporosis can be suggested by X-rays and confirmed by tests to measure bone density. Treatments for osteoporosis, in addition to prescription osteoporosis medications, include stopping use of alcohol and cigarettes, and assuring adequate exercise, calcium, and vitamin D. X-ray of bones will provide whether you have osteoporosis or not. Vitmain D/calcium 1000mg per day (daily) is good for osteoporosis.
http://www.medicinenet.com
Osteopetrosis : Osteopetrosis refers to a group of rare, inherited skeletal disorders characterized by increased bone density and abnormal bone growth. At least one of at least ten genes are involved in developing osteopetrosis. Symptoms and severity can vary greatly, ranging from neonatal onset with life-threatening complications (such as bone marrow failure) to the incidental finding of osteopetrosis on X-ray. Depending on severity and age of onset, features may include fractures, short stature, compressive neuropathies (pressure on the nerves), hypocalcemia with attendant tetanic seizures, and life-threatening pancytopenia. In rare cases, there may be neurological impairment or involvement of other body systems. Osteopetrosis may be caused by mutations in at least 10 genes. Inheritance can be autosomal recessive, autosomal dominant, or X-linked recessive with the most severe forms being autosomal recessive. Management depends on the specific symptoms and severity and may include vitamin D supplements, various medications, and/or surgery. Adult osteopetrosis requires no treatment by itself, but complications may require intervention https://rarediseases.info.nih.gov
Congenital osteopetrosis is a group of disorders resulting in decreased osteoclastic function and hence decreased bone resorption. The accumulation of sclerotic bone compromises marrow space and cranial-nerve foramina and predisposes patients to pathologic fractures. Most patients become blind or anemic before six months of age and die because of poor resistance to infection, neurologic deficits, or bone marrow failure during the first decade — often the first two years — of life.1-4 Bone marrow transplantation is curative therapy,5 but an acceptable donor can be found for only about 40 percent of patients. Overall survival 23 months after transplantation was 47 percent in a European study, and 62 percent of the survivors were considered to have been cured.4 High-dose calcitriol therapy has been reported to ameliorate osteopetrosis in 25 percent of patients. http://www.nejm.org/; Minglin Ou, , Xiaoqing Zhan ,Xiang Chen, Kun Zhang http://www.nature.com/
Williams disease; Clinical characteristics.
Xavier Game, M.D. Jalesh Panicker, M.R.C.P.etal
Williams syndrome (WS) is characterized by cardiovascular disease (elastin arteriopathy, peripheral pulmonary stenosis, supravalvar aortic stenosis, hypertension), distinctive facies, connective tissue abnormalities, intellectual disability (usually mild), a specific cognitive profile, unique personality characteristics, growth abnormalities, and endocrine abnormalities (hypercalcemia, hypercalciuria, hypothyroidism, and early puberty). Feeding difficulties often lead to failure to thrive in infancy. Hypotonia and hyperextensible joints can result in delayed attainment of motor milestones. https://www.ncbi.nlm.nih.gov
Clinical diagnostic criteria are available for Williams syndrome; however, the mainstay for diagnosis is detection of the contiguous gene deletion of the Williams-Beuren syndrome critical region (WBSCR) that encompasses the elastin gene (ELN). More than 99% of individuals with the clinical diagnosis of WS have this contiguous gene deletion, which can be detected using fluorescent in situ hybridization (FISH) and/or deletion/duplication testing. Williams syndrome is transmitted in an autosomal dominant manner.
- Cardiovascular disease (elastin arteriopathy). Any artery may be narrowed. Supravalvar aortic stenosis (SVAS) is the most clinically significant and most common cardiovascular finding; it occurs in 75% of affected individuals. Peripheral pulmonic stenosis (PPS) is common in infancy.
- Distinctive facies. Broad forehead, bi-temporal narrowing, periorbital fullness, a stellate/lacy iris pattern strabismus, short nose, broad nasal tip, malar flattening, long philtrum, thick vermilion of the upper and lower lips, wide mouth, malocclusion, small jaw, and large ear lobes are observed at all ages . Young children have epicanthal folds, full cheeks, and small, widely spaced teeth, while adults typically have a long face and neck, accentuated by sloping shoulders, resulting in a more gaunt appearance .
- Connective tissue abnormalities. Hoarse voice, inguinal/umbilical hernia, bowel/bladder diverticulae, rectal prolapse, joint limitation or laxity, and soft, lax skin are observed.
- Intellectual disability. Most individuals have some degree of intellectual disability, which can range from severe to mild. Some have average intelligence.
- Specific cognitive profile. Strengths in verbal short-term memory and language and extreme weakness in visuospatial construction are typical. The Williams syndrome cognitive profile is independent of IQ.
- Unique personality. Overfriendliness, empathy, generalized anxiety, specific phobias, and attention deficit disorder are commonly observed.
- Growth abnormalities. The growth pattern is characterized by: prenatal growth deficiency, failure to thrive in infancy (70%), poor weight gain and linear growth in the first four years; a rate of linear growth that is 75% of normal in childhood; and a brief pubertal growth spurt. The mean adult height is below the third centile.
- Endocrine abnormalities. Findings include: idiopathic hypercalcemia, hypercalciuria, hypothyroidism, and early (but not precocious) puberty. An increased frequency of subclinical hypothyroidism, abnormal oral glucose tolerance tests, and diabetes mellitus is observed in adults with WS.
Urodynamic testing shows that the most common abnormalities are detrusor over activity, detrusor–sphincter dyssynergia, and upper tract changes.2 Similar to observations in myelomeningocele, urinary tract changes may be detected in utero as well.3 It seems likely that the bladder diverticula and recurrent infections are secondary to detrusor overactivity and detrusor–sphincter dyssynergia; although the underlying mechanisms remain unknown, there may be an association between increased detrusor pressure and an abnormal bladder matrix.4 Associated Chiari malformation can also predispose to neurogenic bladder dysfunction. Of clinical relevance, bladder dysfunction is amenable to treatment, and early diagnosis and commencement of adapted management could prevent complications and improve quality of life. http://www.nejm.org/ http://www.nejm.org/
Trysomi21; Children with Down's syndrome have multiple malformations, medical conditions and cognitive impairment because of the presence of extra genetic material from chromosome 21. The phenotype is variable and the degree of cognitive impairment may be mild, moderate or severe. Children with Down's syndrome often function more effectively in social situations than would be predicted on the basis of their cognitive assessment results. The most common cause is the additional copy of an entire chromosome 21. In about 88% of cases, the extra copy is maternally derived, through an error in cell division called non-disjunction. The extra chromosomal content can occur through different mechanisms and at different points during the formation of germ cells. Non-disjunction can occur during meiosis or from a mitotic error.
Down's syndrome can also occur when only a segment of chromosome 21 has three copies (partial trisomy) or when the whole chromosome is triplicated but only a proportion of the cells are trisomic (mosaicism) with other cells being normal. Mosaicism is found in about 1.3-5% of cases but it is possible that mosaicism occurs more frequently.
Translocation is another mechanism leading to Down's syndrome. Some of the genetic material from chromosome 21, usually from the long arm, is moved to chromosome 14 or 22, or from the long to the short arm of chromosome 21. Translocation occurs in about 4% of cases of Down's syndrome. http://patient.info
Translocation is another mechanism leading to Down's syndrome. Some of the genetic material from chromosome 21, usually from the long arm, is moved to chromosome 14 or 22, or from the long to the short arm of chromosome 21. Translocation occurs in about 4% of cases of Down's syndrome.
Sickle cell anaemia; Over the past century, great advances have been made in the understanding and treatment of sickle cell disease (SCD). This first “molecular disease,” caused by a single gene mutation, has advanced the field of modern human molecular biology. Many important discoveries have been made, and some treatments developed. http://www.scdcoalition.org SCD is a chronic disease that has been neglected for far too long. Those affected by this disease are among the most vulnerable and underserved, and the disease has a profound impact on their lives. Currently, the only approved drug for adults with SCD – hydroxyurea – reduces the severity and frequency of painful episodes and is used for stroke prevention, but may not prevent acute complications or reverse organ damage that can result in early death or other health problems which affect their quality of life (i.e., comorbidities).
SCD was once associated with early childhood mortality, but today in the United States more than 90 percent of people with SCD live into adulthood, which poses new issues and challenges.25 There is only one medication, hydroxyurea, currently approved by the FDA to treat adults with SCD.26 In the United States, there are currently 36 clinical trials underway related to SCD to evaluate novel agents and approaches. This includes several trials involving bone marrow transplantation and gene therapy, which are potentially curative. http://www.scdcoalition.org
Scleroderma; Scleroderma is a long term autoimmune disease that results in hardening of the skin. In the more severe form, it also affects internal organs. The cause is unknown. The underlying mechanism involves the body's immune system attacking healthy tissues.[1] There is a strong association with certain mutations in HLA genes. Environmental factors have also been implicated.
two main types of the disease: the localized form (called localized scleroderma, limited scleroderma, or morphea) and the systemic form (called systemic scleroderma, diffuse scleroderma, generalized scleroderma, or systemic sclerosis). https://en.wikipedia.org
Cystic fibrosis; Cystic fibrosis (CF) is a multisystem disorder caused by mutations of the cystic fibrosis transmembrane conductance regulator (CFTR) gene, located on chromosome 7. Pulmonary disease remains the leading cause of morbidity and mortality in patients with CF. http://www.uptodate.com/ Wolfgang Kamin | Astrid Schwabe | Irene Krämer.
Long-involved in clinical trials for agents to treat cystic fibrosis (CF), Patricia Joseph, MD, professor of medicine and director of Cystic Fibrosis program in the department of internal medicine, University of Cincinnati (UC) Medical Center, says that therapies targeted at different cystic fibrosis (CF) defects are “the most exciting developments in CF right now.” There are five different classes of gene defects in CF, all of which alter the chloride and sodium content of the mucous layer in the airways of the lung. “Because no single CFTR [CF transmembrane conductance regulator]-modulating therapy works for all defects, therapies must be targeted to the specific gene mutation,” says Dr. Joseph.
According to Dr. Joseph, “The modulator drugs are the first to show long-term benefit.” Currently available therapies include potentiator therapy, such as ivacaftor, which corrects the defect in chloride transport and allows the ion channel to open; and corrector drugs, such as lumacaftor-ivacaftor, which help process the CFTR protein and transport it to the cell membrane.
New Drug Agents for Cystic Fibrosis
The recently approved agents, ivacaftor and lumacaftor-ivacaftor, slow CF disease progression, improve their quality of life and may prolong survival. Ivacaftor is approved for the 4% to 5% of CF patients who have the G551D mutation,1 and lumacaftor-ivacaftor is indicated for CF patients with two copies of Phe508del, the most common CF mutation that is present in 45% of CF patients, now numbering over 30,000 individuals in the U.S
Alzheimer disease: Like all types of dementia, Alzheimer's is caused by brain cell death. It is a neurodegenerative disease, which means there is progressive brain cell death that happens over a course of time. The total brain size shrinks with Alzheimer's - the tissue has progressively fewer nerve cells and connections. Apr 29, 2016. Death of brain cells causes memory loss and cognitive decline. A neurodegenerative type of dementia, the disease starts mild and gets progressively worse. Progressive Brain cell death shrinks brain size- the tissue has progressively fewer nerve cells and connections.
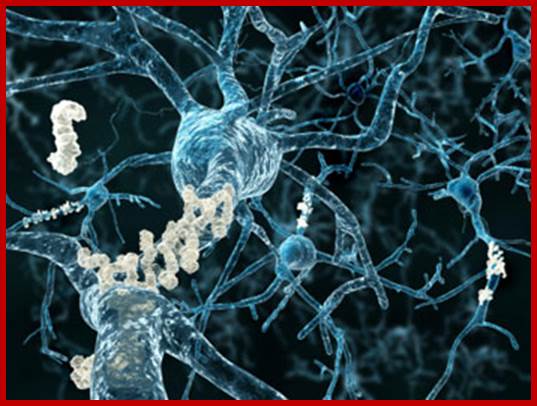
http://www.medicalnewstoday.com
Here is another view of how massive cell loss changes the whole brain in advanced Alzheimer's disease. This slide shows a crosswise "slice" through the middle of the brain between the ears.
In the Alzheimer's brain:
- The cortex shrivels up, damaging areas involved in thinking, planning and remembering. Shrinkage is especially severe in the hippocampus, an area of the cortex that plays a key role in formation of new memories. Ventricles (fluid-filled spaces within the brain) grow larger.
§ Alzheimer's is the most common form of dementia, a general term for memory loss and other intellectual abilities serious enough to interfere with daily life. Alzheimer's disease accounts for 60 to 80 percent of dementia cases. Plaques are found between the dying cells in the brain - from the build-up of a protein called beta-amyloid (you may hear the term "amyloid plaques").
§ The tangles are within the brain neurons - from a disintegration of another protein, called tau.
The cognitive decline is in at least TWO of the five symptom areas listed below (from guidelines jointly produced by the National Institute on Aging and the Alzheimer's Association):
1. Worsened ability to take in and remember new information, for example:
§ "Repetitive questions or conversations
§ Misplacing personal belongings
§ Forgetting events or appointments
§ Getting lost on a familiar route."
2. Impairments to reasoning, complex tasking, exercising judgment:
§ "Poor understanding of safety risks
§ Inability to manage finances
§ Poor decision-making ability
§ Inability to plan complex or sequential activities."
3. Impaired visuospatial abilities (but not, for example, due to eye sight problems):
§ "Inability to recognize faces or common objects or to find objects in direct view
§ Inability to operate simple implements, or orient clothing to the body."
4. Impaired speaking, reading and writing:
§ "Difficulty thinking of common words while speaking, hesitations
§ Speech, spelling, and writing errors."
5. Changes in personality and behavior, for example:
§ Out-of-character mood changes, including agitation; less interest, motivation or initiative; apathy; social withdrawal
§ Loss of empathy
§ Compulsive, obsessive or socially unacceptable behavior.
§ In 2010, some 4.7 million people of 65 years of age and older were living with Alzheimer's disease in the US. The Alzheimer's Association says it accounts for between 60% and 80% of all cases of dementia.
Some are preventable or modifiable factors (for example, reducing the risk of diabetes or heart disease may in turn cut the risk of dementia).
§ Having a certain gene (the apolipoprotein E or APOE gene) puts a person, depending on their specific genetics, at three to eight times more risk than a person without the gene. Numerous other genes have been found to be associated with Alzheimer's disease, even recently (see developments below).7
People with rosacea appear to have a slightly higher risk of developing dementia, and Alzheimer's disease in particular, compared with people without the common chronic inflammatory skin condition.
· People who sleep longer twice as likely to develop dementia.
· Low levels of glucose in the brain may trigger Alzheimer's.
· Calcium imbalance within brain cells may trigger Alzheimer's disease.
· A fifth of dementia cases may be caused by air pollution, study suggests.
· Vitamin A deficiency may cause Alzheimer's to begin in the womb.
· 'Helper cells' can turn toxic in brain injury and diseases.
· Dementia risk reduced by eating 'five-a-day'.
· Alzheimer's disease: Molecular study clarifies potential link to high blood sugar.
There must be memory loss and an impairment in one other area of cognition for a diagnosis of dementia such as Alzheimer's to be made. These criteria also need to be progressive (a worsening compared with how the person has been before), and severe enough to affect daily activities.
§ What is your age?
§ What is the time, to the nearest hour?
§ Repeat an address at the end of the test that I will give you now (e.g. "42 West Street")
§ What is the year?
§ What is the name of the hospital or town we are in?
§ Can you recognize two people (e.g. the doctor, nurse, home help, etc.)?
§ What is your date of birth?
§ In what year did World War 1 begin? (Other widely known dates in the past can be used.)
§ Name the president/prime minister/monarch.
§ Count backwards from 20 down to 1.
A gene known as the APOE-e4 is associated with higher chances of people over the age of 55 years developing Alzheimer's.
Drug therapy
There are no disease-modifying drugs available for Alzheimer's disease but some options may reduce its symptoms and help improve quality of life. There are four drugs in a class called cholinesterase inhibitor approved for symptomatic relief in the US.
§ Donepezil (brand name Aricept)
§ Alantamine (Reminyl)
§ Rivastigmine (Exelon)
§ Tacrine (Cognex).
A different kind of drug, memantine (Namenda), an NMDA receptor antagonist, may also be used, alone or in combination with a cholinesterase inhibitor.
It could be inflammation in the brain that drives Alzheimer's. It suggests blocking a protein that regulates immune cells could be a way to stop the brain-wasting disease. Immune system gone –wrong. Any kind of exercise can improve brain volume and cut the risk of Alzheimer's disease by 50%. Blueberries could be used to fight Alzheimer's, researchers suggest.
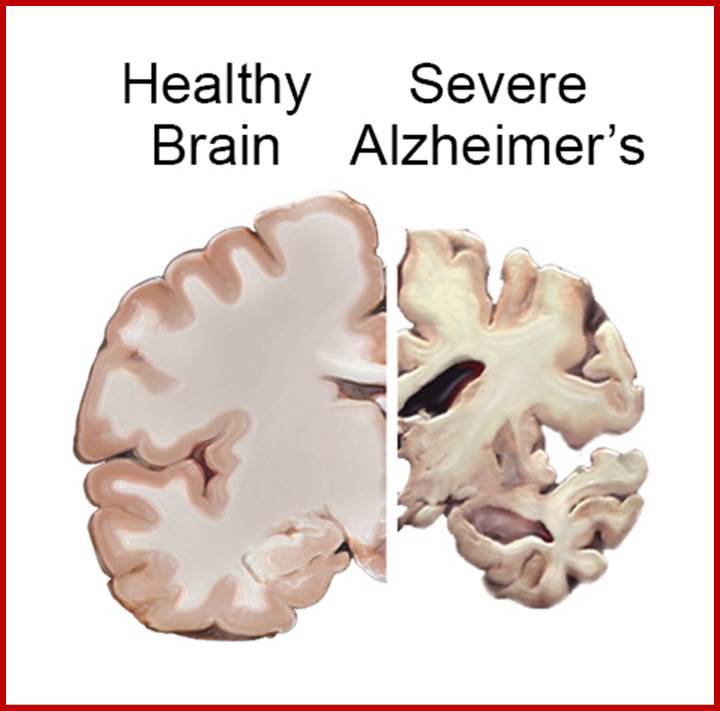
Cross sections of the brain show atrophy, or shrinking, of brain tissue caused by Alzheimer's disease. https://www.nia.nih.gov
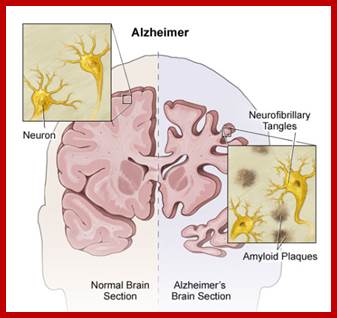
http://www.hopkinsmedicine.org
· Impaired memory, thinking, and behavior; Confusion- Fiber tangles within nerve cells (neurofibrillary tangles); Clusters of degenerating nerve endings (neuritic plaques).
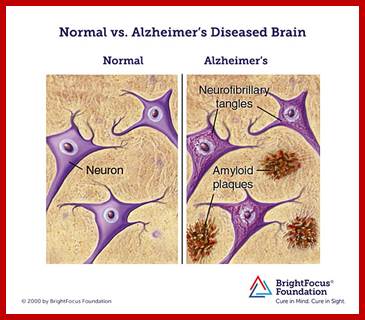
http://www.brightfocus.org The formation of amyloid plaques and neurofibrillary tangles are thought to contribute to the degradation of the neurons (nerve cells) in the brain and the subsequent symptoms of Alzheimer's disease.
AD has been identified as a protein misfolding disease due to the accumulation of abnormally folded amyloid beta protein in the brains of Alzheimer's patients. Amyloid beta, also written Aβ, is a short peptide that is an abnormal proteolytic byproduct of the transmembrane proteinamyloid precursor protein (APP), whose function is unclear but thought to be involved in neuronal development. The presenilins are components of proteolytic complex involved in APP processing and degradation https://en.wikipedia.org
Alzheimer's disease has been identified as a protein misfolding disease, or proteopathy, due to the accumulation of abnormally folded Amyloid-beta proteins in the brains of AD patients. Abnormal amyloid-beta accumulation can first be detected using cerebrospinal fluid analysis and later using positron emission tomography (PET).
Abnormal amyloid-beta accumulation can first be detected using cerebrospinal fluid analysis and later using positron emission tomography (PET). Although AD shares pathophysiological mechanisms with prion diseases, it should be noted that AD is not transmissible like prion diseases.[16] Amyloid-beta, also written Aβ, is a short peptide that is a proteolytic byproduct of the transmembrane protein amyloid precursor protein (APP), whose function is unclear but thought to be involved in neuronal development. The presenilins are components of a proteolytic complex involved in APP processing and degradation. Although amyloid beta monomers are harmless, they undergo a dramatic conformational change at sufficiently high concentration to form a beta sheet-rich tertiary structure that aggregates to form amyloid fibrils that deposit outside neurons in dense formations known as senile plaques or neuritic plaques, in less dense aggregates as diffuse plaques, and sometimes in the walls of small blood vessels in the brain in a process called amyloid angiopathy or congophilic angiopathy.
AD is also considered a tauopathy due to abnormal aggregation of the tau protein, a microtubule-associated protein expressed in neurons that normally acts to stabilize microtubules in the cell cytoskeleton. Like most microtubule-associated proteins, tau is normally regulated by phosphorylation; however, in AD patients, hyperphosphorylated tau accumulates as paired helical filaments that in turn aggregate into masses inside nerve cell bodies known as neurofibrillary tangles and as dystrophic neurites associated with amyloid plaques.
The Levels of the neurotransmitter acetylcholine are reduced. The levels of neurotransmitters serotonin, norepinephrine, and somatostatin are also often reduced. Glutamate levels are usually elevated.
A mechanism for neurotoxicity has been proposed based on the loss of microtubule-stabilizing tau protein that leads to the degradation of the cytoskeleton. However, consensus has not been reached on whether tau hyperphosphorylation precedes or is caused by the formation of the abnormal helical filament aggregates. Support for the tau hypothesis also derives from the existence of other diseases known as tauopathies in which the same protein is identifiably misfolded. However, a majority of researchers support the alternative hypothesis that amyloid is the primary causative agent.
The amyloid hypothesis is initially compelling because the gene for the amyloid beta precursor APP is located on chromosome 21, and patients with trisomy 21 - better known as Down syndrome - who thus have an extra gene copy almost universally exhibit AD-like disorders by 40 years of age
Abnormally low rates of cerebral glucose metabolism are found in a characteristic pattern in the Alzheimer’s disease brain, particularly in the posterior cingulate, parietal, temporal, and prefrontal cortices. These brain regions are believed to control multiple aspects of memory and cognition. This metabolic pattern is reproducible and has even been proposed as a diagnostic tool for Alzheimer’s disease. Moreover, diminished cerebral glucose metabolism (DCGM) correlates with plaque density and cognitive deficits in patients with more advanced disease.
The apolipoprotein E gene (APOE4, a genetic risk factor for Alzheimer’s disease), diminished cerebral glucose metabolism (DCGM) ; Interestingly, a connection has been established between Alzheimer's disease and diabetes during the past decade, as insulin resistance, which is a characteristic hallmark of diabetes, has also been observed in brains of subjects suffering from Alzheimer's disease. Neurotoxic oligomeric amyloid-β species decrease the expression of insulin receptors on the neuronal cell surface and abolish neuronal insulin signaling. It has been suggested that neuronal gangliosides, which take part in the formation of membrane lipid microdomains, facilitate amyloid-β-induced removal of the insulin receptors from the neuronal surface.[55] In Alzheimer's disease, oligomeric amyloid-β species trigger TNF-α signaling. c-Jun N-terminal kinase activation by TNF-α in turn activates stress-related kinases and results in IRS-1 serine phosphorylation, which subsequently blocks downstream insulin signaling. The resulting insulin resistance contributes to cognitive impairment. Consequently, increasing neuronal insulin sensitivity and signaling may constitute a novel therapeutic approach to treat Alzheimer's disease
At late stages of the disease, insoluble plaques composed of the misfolded chains of a protein fragment called amyloid β (Aβ) accumulate in the patient’s brain. However, recent research has indicated that smaller Aβ aggregates, which are soluble in the intercellular fluid and may form at an earlier stage, are the most toxic form of the peptide. These toxic Aβ oligomers may infest the brain long before large-scale brain damage becomes apparent. It has been established that the particles interact strongly with cell membranes, possibly causing nerve cells to leak. The leaky membranes are unable to maintain the difference of ion concentration between the inside and outside of cells that is essential to information processing, and consequently to central nervous system function. http://www.cmu.edu
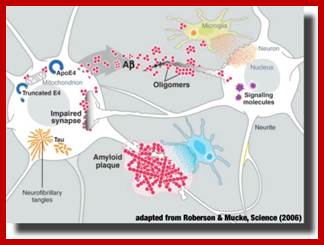
The Alzheimer’s peptide Aβ is a natural cleavage product released within neuronal cells. If misfolded, Aβ forms distinct structures that are prone to aggregation, first into oligomers, then into progressively larger structures called amyloid plaques. It is now believed that Aβ oligomers are the toxic form of the peptide that may impair basic neuronal processes, such as the communication between the nerve cells, long before clinical symptoms become obvious. However, molecular details of these early processes remain largely unknown.
Alzheimer's worsens over time. Alzheimer's is a progressive disease, where dementia symptoms gradually worsen over a number of years. In its early stages, memory loss is mild, but with late-stage Alzheimer's, individuals lose the ability to carry on a conversation and respond to their environment. Alzheimer's is the sixth leading cause of death in the United States. Those with Alzheimer's live an average of eight years after their symptoms become noticeable to others, but survival can range from four to 20 years, depending on age and other health conditions. Learn more: 10 Warning Signs and Stages of Alzheimer's Disease.
1. We have purified and characterized the cerebral amyloid protein that forms the plaque core in Alzheimer disease and in aged individuals with Down syndrome. The protein consists of multimeric aggregates of a polypeptide of about 40 residues (4 kDa). The amino acid composition, molecular mass, and NH2-terminal sequence of this amyloid protein are almost identical to those described for the amyloid deposited in the congophilic angiopathy of Alzheimer disease and Down syndrome, but the plaque core proteins have ragged NH2 termini. The shared 4-kDa subunit indicates a common origin for the amyloids of the plaque core and of the congophilic angiopathy. There are superficial resemblances between the solubility characteristics of the plaque core and some of the properties of scrapie infectivity, but there are no similarities in amino acid sequences between the plaque core and scrapie polypeptides. http://www.pnas.org C L Masters, G Simms, et al.
100 billion nerve cells. 100 trillion synapses. dozens of neurotransmitters. This "strength in numbers" provides your brain's raw material. Over time, our experiences create patterns in signal type and strength. These patterns of activity explain how, at the cellular level, our brains code our thoughts, memories, skills and sense of who we are.
The positron emission tomography (PET) scan on the left shows typical patterns of brain activity associated with:
Reading words, Hearing words, Thinking about words and Saying words
Activity is highest in red areas and then decreases through the other colors of the rainbow from yellow to blue-violet. Specific activity patterns change throughout life as we meet new people, have new experiences and acquire new skills. The patterns also change when Alzheimer's disease or a related disorder disrupts nerve cells and their connections to one another.
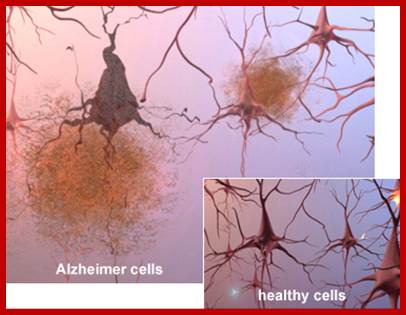
http://www.alz.org/
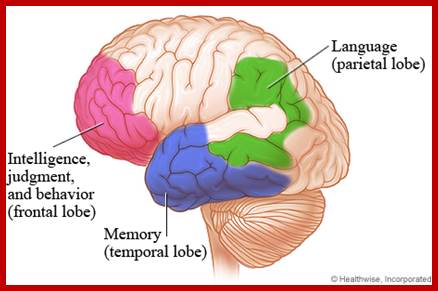
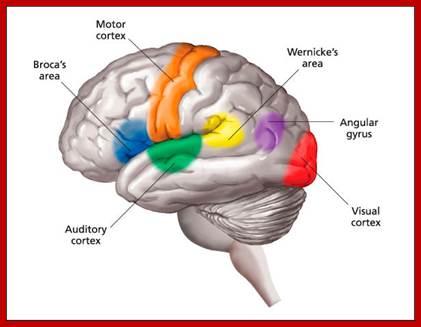
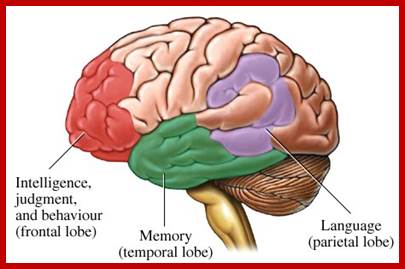
http://southcountychildandfamily.com
Here is another view of how massive cell loss changes the whole brain in advanced Alzheimer's disease. This slide shows a crosswise "slice" through the middle of the brain between the ears. In the Alzheimer's brain: the cortex shrivels up, damaging areas involved in thinking, planning and remembering. Shrinkage is especially severe in the hippocampus, an area of the cortex that plays a key role in formation of new memories. Ventricles (fluid-filled spaces within the brain) grow larger; http://www.alz.org
Alzheimer's is the most common form of dementia, a general term for memory loss and other intellectual abilities serious enough to interfere with daily life. Alzheimer's disease accounts for 60 to 80 percent of dementia cases.
Dementia is caused by damage to brain cells. This damage interferes with the ability of brain cells to communicate with each other. When brain cells cannot communicate normally, thinking, behavior and feelings can be affected. The brain has many distinct regions, each of which is responsible for different functions (for example, memory, judgment and movement). When cells in a particular region are damaged, that region cannot carry out its functions normally.
Alzheimer's
worsens over time. Alzheimer's
is a progressive disease, where dementia symptoms gradually worsen over a
number of years. In its early stages, memory loss is mild, but with late-stage
Alzheimer's, individuals lose the ability to carry on a conversation and
respond to their environment. Alzheimer's is the sixth leading cause of death
in the United States. Those with Alzheimer's live an average of eight years
after their symptoms become noticeable to others, but survival can range from
four to 20 years, depending on age and other health conditions.
Learn more: 10 Warning Signs and Stages of Alzheimer's Disease- Memory lass disrupting daily life, Planning becomes
difficult, not possible complete the work that is thought of or difficult
remember what has been thought to do, Confusion of distinguishing people or
remembering friends/relatives, time or place, trouble in understanding visual
images, problems in using words during speaking, misplacing things and losing
the ability to retrace or recall, Decrease in proper judgement , with drawls
from social activities, changes in moods and personality.
Calmodulin:

https://en.wikipedia.org/ Calmodulin
Calmodulin is a small, highly conserved protein that is 148 amino acids long (16.7 KDa). The protein has two approximately symmetrical globular domains each containing a pair of EF-hand motifs (the N- and C-domain) separated by a flexible linker region for a total of four Ca2+ binding sites.
The protein has two approximately symmetrical globular domains each containing a pair of EF-hand motifs (the N- and C-domain) separated by a flexible linker region for a total of four Ca2+ binding sites. Each EF-hand motif allows calmodulin to sense intracellular calcium levels by binding one Ca2+ ion. Calcium ion binding regions are found in the following positions in the sequence of amino acids: 21-32, 57-68, 94-105 and 130-141; each region that calcium binds to is exactly 12 amino acids long. These regions are located between two alpha helices in the EF-hand motifs, the first two regions (21-32 and 57-68) are on one side of the linker region the other two (94-105 and 130-141) are on the other side.
Calmodulin's structure is very similar to the structure of Troponin C (which is another calcium binding protein). They are both members of the EFh superfamily. Troponin C, like Calmodulin, has two globular domains that are connected by a linker region. However, Troponin C and Calmodulin differ in the length of the linker region; the linker region of Calmodulin is smaller than that of Troponin C.
https://en.wikipedia.org
Role in metabolism;
Calmodulin plays an important role in the activation of , which ultimately leads to glucose being cleaved from glycogen by glycogen phosphorylase, phosphoryl kinase
Calmodulin also plays an important role in lipid metabolism by affecting Calcitonin. Calcitonin is a polypeptide hormone that lowers blood Ca2+ levels and activates G Protein cascades that leads to the generation of cAMP. The actions of calcitonin can be blocked by inhibiting the actions of calmodulin, suggesting that calmodulin plays a crucial role in the activation of calcitonin.
Role in short-term and long-term memory;]
Long-term potentiation (LTP) requires a depolarization of GABAergic neurons (Neurons that use GABA as a neurotransmitter) in the hippocampus. Calmodulin Kinase II (CaM-K II) plays a crucial role in achieving LTP. It contributes to the phosphorylation of an AMPA receptor which increases the sensitivity of AMPA receptors.[18] Furthermore, research shows that inhibiting CaM-K II interferes with LTP.
Calmodulin family members;
· Calmodulin 1 (CALM1), Calmodulin 2 (CALM2), ‘
· Calmodulin-like 1 (CALML1), Calmodulin-like 3 (CALML3), Calmodulin-like 4 (CALML4), Calmodulin-like 5 (CALML5), Calmodulin-like 6 (CALML6);
- A protein kinase is a kinase enzyme that modifies other proteins by chemically adding phosphate groups to them (phosphorylation). The human genome contains about 500 protein kinase genes and they constitute about 2% of all human genes. Up to 30% of all human proteins may be modified by kinase activity, and kinases are known to regulate the majority of cellular pathways, especially those involved in signal transduction. https://en.wikipedia.org; CAMK, also written as CaMK, is an abbreviation for the Ca2+/calmodulin-dependent protein kinase class of enzymes. CAMKs are activated by increases in the concentration of intracellular calcium ions (Ca2+) and transfers phosphates from ATP to defined serine or threonine residues in other proteins. Activated CAMK is involved in the phosphorylation of transcription factors and therefore, in the regulation of expression of responding genes. Members of this enzyme class include;
CAMKI, CAMKIα, CAMKIβ, CAMKIδ’ CAMKIγ, CAMKII[3], CAMKIIα, CAMKIIβ, CAMKIIδ, CAMKIIγ, CAMKIII, CAMKIV
Human Somatogen:
Hunter Syndrome: Hunter syndrome is one type of a group of inherited metabolic disorders called mucopolysaccharidoses (MPSs), and Hunter syndrome is referred to as MPS II. The syndrome symptoms vary and range from mild to severe. Symptoms aren't present at birth, but often begin around ages 2 to 4.
Hunter syndrome develops when a defective chromosome is inherited from the child's mother. Because of that defective chromosome, an enzyme that's needed to break down complex sugar molecules is missing or malfunctioning. Without this enzyme, massive amounts of these complex sugar molecules collect in the cells, blood and connective tissues, causing permanent and progressive damage.
Hunter syndrome is what's known as an X-linked recessive disease. This means that women carry the defective disease-causing X chromosome and can pass it on, but women aren't affected by the disease themselves.
Hunter syndrome is what's known as an X-linked recessive disease. This means that women carry the defective disease-causing X chromosome and can pass it on, but women aren't affected by the disease themselves.
Sex. Hunter syndrome nearly always occurs in males. Girls are far less at risk of developing this disease because they inherit two X chromosomes. If one of the X chromosomes is defective, their normal X chromosome can provide a functioning gene. If the X chromosome of a male is defective, however, there isn't another normal X chromosome to compensate for the problem.
Signs and symptoms may include: An enlarged head (macrocephaly), Thickening of the lips, A broad nose and flared nostrils, A protruding tongue, A deep, hoarse voice, Abnormal bone size or shape and other skeletal irregularities, A distended abdomen, as a result of enlarged internal organs, Diarrhea, White skin growths that resemble pebbles, Joint stiffness, Aggressive behavior, Stunted growth, Delayed development, such as late walking or talking.
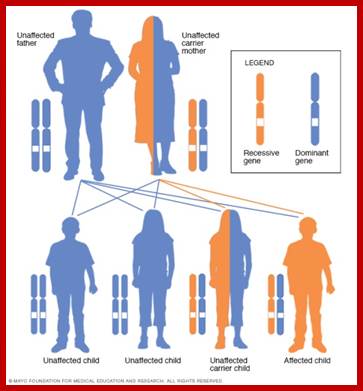
http://www.mayoclinic.org/ Mayo Clinic Staff
Some treatments that are in their early stages of development have had some success by slowing the disease's progress and lessening its severity. Enzyme therapy. This treatment uses man-made or genetically engineered enzymes to replace your child's missing or defective enzymes and ease the disease symptoms. This treatment is given once a week through an intravenous (IV) line. Gene therapy. Replacing the chromosome responsible for producing the missing enzyme could theoretically cure Hunter syndrome, but much more research is needed before this therapy will be available.