Replication of Telomeric DNA:
Unlike E.coli DNA, termination of eukaryotic DNA in each of the replicons doesn’t pose any encounters for there are no special structural organizations, for the replication fork to move again into newly formed ds DNA; for replication forks end in the terminal region, no further initiation of replication; it just ends. Replication is not continuous smooth peeling and when it comes to chromosomal ends; it encounters replication pause sites at Telomeric regions. In centromeric region there are replication barriers mediated by DNA structures or repetitive sequences.
· Each of chromosomes in eukaryotes has hundreds of replicons organized in linearly. Termination of each of the replicons is simple.
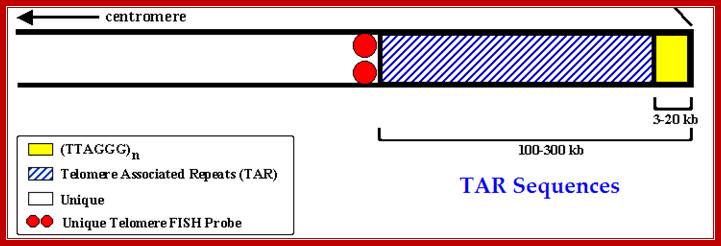
· But when comes to the end of the chromosomes (there are two ends for each chromosome), replication forks at terminal replicons does not proceed for the ends of chromosomal DNA contain free terminals and the free terminals of double stranded DNA have unequal lengths; one longer than the other. In addition the longer strand is looped and inserted to form quadruplex (?) structure or loops with ends of DNA partially base paired. This region of the chromosomal DNA is called Telomere. Replication of this free ends of DNA poses a problem because, if primers at the end of lagging strand are removed there is no primer to extend and complete the last stretch of 3’ end of the strands, because the last primer 5’>3’ at each of the two newly formed strands is the last segment and it is removed by exonucleases like Fen and its related proteins. The ends of dsDNA strands remain open exposed to exonucleases, but protected by loop formation and telomere specific protein binding.
· If the primers are removed there is no direct way to fill up the gaps.

The picture shows fluorescent labeled telomeric ends; http://www.laskerfoundation.org/
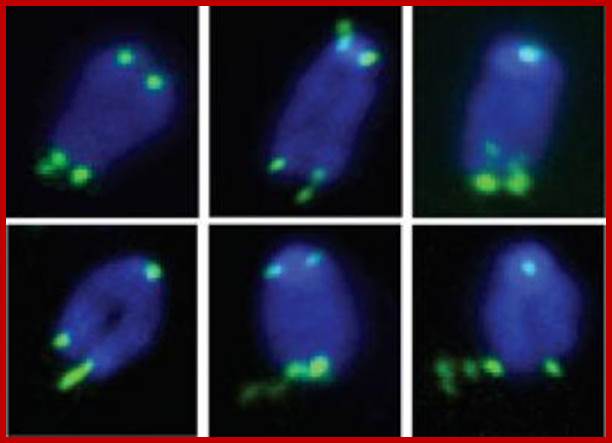
Fragile telomeres. Handle with Care-This is a series of images showing chromosomes with fragile telomeres (green). Without the protein TRF1, telomeres resemble common fragile sites, unstable regions on chromosomes that break into segments or stretch due to faulty DNA replication. Credit: Cell.; Titia de Lange, head of the Laboratory of Cell Biology and Genetics Rockefeller University
Many linear genomic DNAs have developed strategies to replicate and protect the ends from exonuclease digestion. In Adenovirus ADV, the 5’ ends are bound by a 50 KD protein, which not only protect the ends, but it is also used for initiation of replication. Few other linear viral DNAs show self-complementary structure. So they are folded and the free ends are protected. Examples-Chlorella viral DNA, some Archaeal viral genomes, EBV viral genome, human Herpes virus and many others. In the case of Picorna RNA genome, it is protected with VPg protein at their ends.
· During chromosomal replication, Euchromatin DNA is replicated earlier, the centromeric DNA is replicated later and the last to be replicated is telomeric DNA.
The ends of eukaryotic chromosomes are noted as specialized structures of chromosomes called telomeres; they appear as fine granular structures (if stained and observed under high resolution microscope). High-resolution light microscopes have delineated their distinctive features. Their behavior has been observed by genetic studies.
· Telomeres have been known to prevent fusion of wrong ends of broken chromosomes.
They are found to provide stability to chromosomes, what centromere does to chromosomes.
· Replication shortens the chromosome. The body’s natural cure to this dilemma is the production of expandable nucleotides chain at the 3’ end of every chromosome. These "cannon fodder" nucleotide threads are called telomeres. Telomeres are repetitive hexameric (6 base pair) sequences of DNA. In humans this repeated G-rich sequence is AGGGTT. These sequences are 1000-1700 base- pairs long at the beginning. Cells seldom survive past about 50 divisions in vitro, which most researchers ascribe to the deletion of too many genes in the process of replication.
· Does the fountain of youth spring from our chromosomes?
· Telomeres have been implicated in aging, for aged cells have very short telomeric sequences in comparison to young proliferating cells, which have longer and stable sequences.
Telomeres are known to have specialized structures having their own composition, structure and behavior. They don’t have any coding sequences for any known proteins.
· Telomeric regions contain DNA with distinctive features of its own.
Telomeric DNA has specific short sequences of tandem repeats, ranging from few repeats to several hundred or more (a total length of 5000 to 10000 or more base pairs).
· The DNA ends are not free but folded into closed loops, with the insertion of 3’ end single stranded region; which can form a single loop recognizable under TEM, or they may develop quartet or quadruplex structural form; many such loops may be found at each ends.
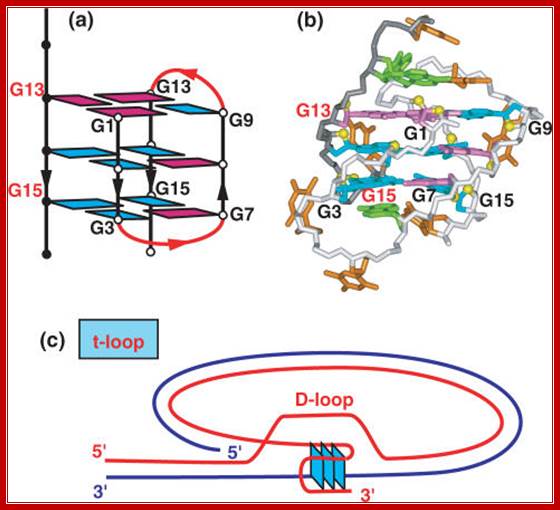
NMR based (a) (3 + 1) folding topology and (b) solution structure of the three repeat human telomere bimolecular G quadruplex formed by the d[G3(T2AG3)2T sequence in Na+ solution (coordinates deposition: 2AQY) Nucleic Acids Res. 2007 Dec; 35(22):7429-7455, Patel DJ1, Phan AT, Kuryavyi V.
The ends are protected and stabilized by variety of proteins, such as TRF1 and TRF2, hRAP interacts with TRF2; TRF stands for Telomeric Repeat binding Factors). Telomeres are also associated with Poly-ADP Ribose Polymerase (PARP) or Tankyrase. There may be several telomere-binding proteins and several telomere-capping proteins. The 3’ end of the telomeric DNA bound by Pot1p (give protection of telomeric ends).
· Such proteins have been found to protect viral DNA ends too e.g. Epstein-Barr viral DNA
In yeasts telomere related genes have been identified, called EST (ever shorter telomeres).
· They are p123, and EST-2, EST-3.
Every cycle of chromosomal DNA replication results in the loss of DNA segments at their ends, because of improper replication of the ends.
· There should be components and a process that could compensate for the loss and restore it to its original length or increase its length.
The kind of short sequences found in telomeres is more or less similar in most of the organisms including humans. Some sequences may slightly vary but not much, but the sequences are species specific.
· Tetrahymena 5’ TTGGGG
· Oxytricha 5’TTTTGGGG
· Trypanosome 5’GGGTTT
· Dictyostelium 5’GGGTTT
· Yeast 5’GGT (GT) 1-3
· Arabidopsis 5’G3AT3
· Mouse 5’TTAGGG
· Homo sapiens 5’ TTAGGG3’
Extended List with some repeats:
|
TTAGGG |
||
|
Filamentous fungi |
TTAGGG |
|
|
TTAGGG |
||
|
AG(1-8) |
||
|
Kinetoplastid protozoa |
TTAGGG |
|
|
Ciliate protozoa |
TTGGGG |
|
|
TTGGG(T/G) |
||
|
TTTTGGGG |
||
|
Apicomplexan protozoa |
TTAGGG(T/C) |
|
|
Higher plants |
TTTAGGG |
|
|
TTTTAGGG |
||
|
TTAGG |
||
|
TTAGGC |
||
|
Fission yeasts |
TTAC(A)(C)G(1-8) |
|
|
Budding yeasts |
TGTGGGTGTGGTG
(from RNA template) |
|
|
TCTGGGTG |
||
|
GGGGTCTGGGTGCTG |
||
|
GGTGTACGGATGTCTAACTT- CTT |
||
|
GGTGTA[C/A]GGATGTCACGA- TCATT |
||
|
GGTGTACGGATGCAGACTC- GCTT |
||
|
GGTGTAC |
||
|
GGTGTACGGATTTGATTAGT- TATGT |
||
|
GGTGTACGGATTTGATTAGG- TATGT |
Within the telomeric region, DNA shows some repeated or an array of discontinuities or nicks. This may be due to organization of DNA either in the form of hairpin structures, or quadruplex structure with (G) 4 quartet and Hoogsteen bonding. Such quartet structures produce loops. There can be several such quadruplex loops in each of the Telomeric ends, hence one find discontinuities.
· Replication of DNA of telomeric ends is unique and no other molecular processes can equal though not surpass.
· A complex of proteins and enzymes perform this. The enzyme involved is called Telomerase.
· Many genes involved in this process have been identified.
· The enzyme has been purified and the gene for it has been cloned, and other related genes have also been cloned.
The Telomerase complex consists of a single stranded RNA of ~160 ntds in Tetrahymena with CAACCCCAA, 190 ntds long in Euplotes with CAAAACCCCAAAACC, 450 ntds long in mice with CCUAACCCU and 450ntds long in Humans with CUAACCCUAAC. They have a sequence repeats complementary to the ends of telomeric DNA sequences.
Telomerase enzyme is complex component of chromosomes (Cohen and Dr.Scott, Sydney). Telomerase consists of Genes for Telomerase, two molecules each of human telomerase reverse transcriptase (TERT), telomerase RNA (TR or TERC), and dyskerin (DKC1). The genes of Telomerase TERT, TERC,DKC and TEP1. Genes are localized in different chromosomes. The human TERT gene (hTERT) is translated into a protein of 1132 amino acids. TERT is a reverse transcriptase, it creates single stranded DNA using its carried RNA piece.
The TERT polypeptide folds with (and carries) TERC, a non-coding RNA (451 nucleotides long). TERT has a 'mitten' structure that allows it to wrap around the chromosome to add single-stranded telomere repeats. The protein consists of four conserved domains (RNA-Binding Domain (TRBD), fingers, palm and thumb), organized into a ring configuration that shares common features with retroviral reverse transcriptases, viral RNA polymerases and bacteriophage B-family DNA polymerases.
By using the TERC RNA, TERT can add a six nucleotide repeat sequences 5’-TTAGGG in vertebrates to 3’ strand of chromosome ends. These TTAGGG repeats with their binding proteins are called telomeres. The template sequence of TERC is 3’CASAUCCCAAUC-5’. The enzyme binds to the last Telomere sequences and add a new telomeric repeats 5’-GGTTAG-3’ sequence and then releases from it, and realigns the new 3’ end to the template . The process is repeated many times.
Oftyen the telomeric DNA sequences get shortened due to certain reasons, but the enzyme telomerase reverses telomere shortening and maintains the telomere ends. It the cell divides without the Addition of telomere ends it will reach what is called Hayflick limit. If this continues for 45-70 divisions, cell divisions stop, and lead to cancerous growth. Embryonic stem cells, epidermal cells, activated T cell and B lymphocyte cells. Telomere length does not change with the age. Another study found little evidence that, in humans, telomere length is a significant biomarker of normal aging with respect to important cognitive and physical abilities.
Premature aging syndromes including Werner syndrome, Ataxia telangiectasia, Ataxia-telangiectasia like disorder, Bloom syndrome, Fanconi anemia and Nijmegen breakage syndrome are associated with short telomeres. However, the genes that have mutated in these diseases all have roles in the repair of DNA damage and the increased DNA damage may, itself, be a factor in the premature aging (see DNA damage theory of aging). An additional role in maintaining telomere length is an active area of investigation. When telomeric end DNA gets shortened without replacement it is possible two such chromosomal ends can join or fuse. This leads to unstable genome. Example human chromosome 2 formed where two chromosomes of apes 2a and 2b have joined end to end, 48 chromosomes have reduced to 46 thus humans emerged out of apes, chimps and gorilla group of mammals.
Many cancer cells are considered 'immortal' because telomerase activity allows them to live much longer than any other somatic cell, which, combined with uncontrollable cell proliferation, ex. HeLa cell remain young and active for many generations. This has led to the identification of mutation combinations that form tumorigenic cells in a variety of cell types. While the combination varies by cell type, the following alterations are required in all cases: TERT activation, loss of p53 pathway function, loss of pRb pathway function, activation of the Ras or myc proto-oncogenes, and aberration of the PP2A protein phosphatase. Paradox is that telomerse action is required for the stable chromosomal genome, but cancer cells require telomerase activity. If telomeres ends are not added, as the cell divides, cells either die or lead to abnormalities and die.
Telomeres:
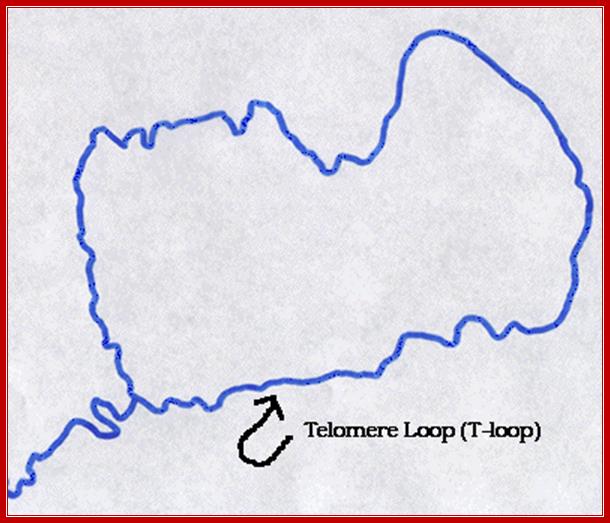
Telomeres are structures at the ends of all linear chromosomes that consist, in mammalian cells, of hexanucleotide repeats [(TTAGGG)n] and many associated proteins . Whereas most of the telomeric DNA is double stranded, a G-rich single strand forms a terminal 3' overhang. Recent evidence indicates that the structure of the telomeric end consists of a unique 'T loop', which is formed by invasion of the single-stranded 3' terminus into double-stranded telomeric DNA58 (see figure, part a). This structure might be important in mediating telomere function by providing a 'cap' at the telomere end that protects against chromosomal instability, end–end fusions and consequent events such as cell death or replicative senescence. Telomeric repeat-binding factor 1 (TRF1) and TRF2 bind to double-stranded telomeric DNA and have a role in telomere stabilization and telomere-length regulation.

Several proteins have been shown to be associated with telomeres (see figure, part b). Some of these proteins bind directly and specifically to telomeric DNA — TRF1 (Ref. 60) and TRF2 (Ref. 61) bind to double-stranded telomeric DNA, and POT1 (protection of telomeres 1) binds to single-stranded 3' terminal telomeric DNA. Other proteins are localized to telomeres through protein–protein interactions3. Several of these proteins have crucial roles in regulating telomere function, either by affecting telomere length or by altering capping function independent of telomere length. Although the mechanisms that mediate these effects are not completely understood, it is intriguing that several proteins that are known to have a role in sensing DNA breaks and in mediating DNA repair — for example, Ku-family members63 and the NBS–MRE11–RAD50 complex — are found at telomeres. The two components of telomerase are illustrated — the RNA template and the catalytic protein telomerase reverse transcriptase (TERT). RAP1, repressor and activator protein 1; TEP1, telomerase-associated protein 1. Richard J. Hodes, Karen S. Hathcock & Nan-ping Weng
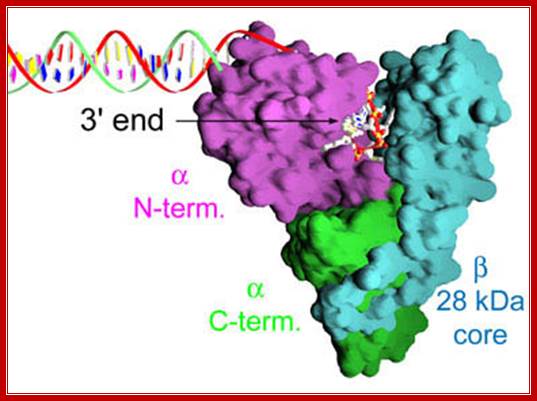
This 3-D model of reverse transcriptase bound ss Telomeric DNA; the enzyme components such as N end and the C-end are shown, while the beta core is shown light blue color. http://www.vegatest.bg
This organism contains 8 x 107 telomeres in its macronucleus and it is, therefore, a rich source of telomerase - each cell contains 3 x 105 molecules. When they determined the amino acid sequence of the p123 component of telomerase, they found that parts of the protein sequence were very similar to an important domain in reverse transcriptase and they modeled the active site of telomerase on the "palm" motif of polymerases.
- Tetrahymena cells carrying a mutation in telomerase die off earlier than usual.
- Human somatic cells have no telomerase. They also have a limited capacity to divide. This may be a critical aspect of the aging process.
- Human germ cells have telomerase. Germ cell chromosomes must be full length!
- Human ovarian carcinoma cells have telomerase activity. The telomeres found in these cells are relatively short - nevertheless, this may explain their capacity for prolonged cell division.
- Some scientists have attempted to express telomerase in cell lines in order to extend their life span. However this may cause the expression of oncogenes and render the cell lines unsuitable for use.
· Each of the telomerase RNAs are specific to it species.
These RNAs contain segments of 15 to 22 bases, as repeats but complementary to telomeric DNA.
· One strand of telomeric DNA, say in Tetrahymena, with 5’TTGGGG3’ sequence is an extended single strand.
The enzyme Telomerase turned out to be a Reverse transcriptase. So this has the ability to produce a cDNA and can switch the template and can copy the strand that itself have synthesized?
· The Telomerase complex uses a part of the RNA to base pair with the 5’ TTGGGG3’ strand and the rest of the RNA acts as the template.
The enzyme uses --TTGGGG3’OH group and extends to produce complementary strand.
· Once the repeats in RNA are completed, the complex switches or slides to the end of the newly synthesized Telomeric DNA.
This way it can repeat several times to generate a long 5’GGGATT3’ strand with repeats of the same many times over. It is believed there are proteins such as Ku 70 and Ku 78 bound at the end of the dsDNA control and regulates the length.
· How the length is controlled in not clear.
This top strand can bend or loop back on its own and base pair to its own template and it can extend to copy the GGGGTT strand or the same RNA 3’ end can be used as the primer to fill the gap.
5’---TTGGGGTTGGGG 3’OH (T-DNA
RNA 3’---AATCCCAATCCCAATCCCAATCCCAATCCC---(n) telomerase--5’
Human telomere DNA:
5’TTAGGGTTAGGGTTAGGGTTAGGGTTAGGGTTAGGG3’
3’AAUCCCAAUCCCAAUCCCAAUCCCAAUCCCAA5’-----’Telomerase RNA”
[3’AAUCUCCCAA] ----5’tel RNA-3’
Replication of Telomeric DNA:
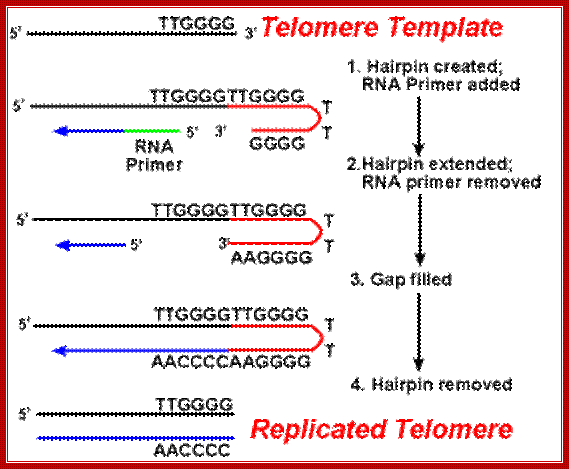
Maintaining proper telomere length is essential for cell survival, thus telomerase is carefully controlled by both positive and negative regulation. These regulatory mechanisms ensure that the shortest telomeres are preferentially elongated by telomerase, and, conversely, that telomerase is repressed at longer telomeres to prevent over-elongation.
Telomerase holoenzyme consists of four entities – the catalytic subunit Est2, the associated RNA component TLC1, and two additional subunits Est1 and Est3, which both participate in telomere length regulation. In the absence of any one of these critical factors, telomeres continually shorten as cells divide until replicative senescence is triggered, giving rise the Est name The Wuttke Lab;http://chem.colorado.edu/

http://chem.colorado.edu/
The primary difficulty with telomeres is the replication of the lagging strand. Because DNA synthesis requires a RNA template (that provides the free 3'-OH group) to prime DNA replication, and this template is eventually degraded, a short single-stranded region would be left at the end of the chromosome. The action of the telomerase enzymes ensure that the ends of the lagging strands are replicated correctly. A well-studied system involves the Tetrahymena protozoa organism. The telomeres of this organism end in the sequence 5'-TTGGGG-3'. The telomerase adds a series of 5'-TTGGGG-3' repeats to the ends of the lagging strand. A hairpin occurs when unusual base pairs between guanine residues in the repeat form. Next the RNA primer is removed, and the 5' end of the lagging strand can be used for DNA synthesis. Ligation occurs between the finished lagging strand and the hairpin. Finally, the hairpin is removed at the 5'-TTGGGG-3' repeat. Thus the end of the chromosome is faithfully replicated. The following figure shows these steps. http://www.ndsu.edu/
· This can be done either by the reverse transcriptase or host DNA polymerase called DNA-pol delta.
But a mechanism has been designed to explain how telomeric complementary strand is synthesized.
· Some of the factors, especially TRF2 binds to the top ss strand and finds ds region a little behind.
The top ss strand intercalates and displaces one of the two strands and pairs with another strand, similar to strand translocation during genetic recombination.
· The intruded strand provides 3’end, which can be used as the primer and it can extend and generate ds structure, but it actually generates a loop called D-Loop.
In the loops, the ends are not ligated, but free, yet not to be accessed by exonucleases.
· The loop is stabilized and protected from exonucleases with the binding of several TRFs.
The loop can be established and stabilized by quartet formation. In this GGGGTT repeats of four segments, the second G in every repeat gets base paired (Hoogsteen base pairing) to give quartet and the D-loop.
· At each of these ends, one may find several such loops, for the telomeric DNA moves very slow in the gel.
5’ GG*GGTTGG*GGTTGG*GGTTGG*GGTT….
G s with astrich mark one repeat base pairs with another thus they form quartets. Such quartets can be formed in the telomeric region and they are bound by specific proteins and their ends are stabilized and also their DNA is repressed by the binding specific proteins.
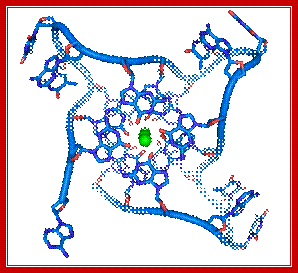
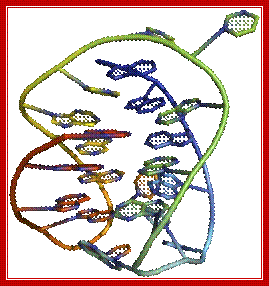
Top figure Parallel telomeric quadruplex structure; http://commons.wikimedia.org/. The bottom figure shows-Schematic representation of G-quadruples structural feature of Telomeric DNA; Lower figure shows the possible 3-D structure of quadruplex DNA; http://www.atdbio.com/
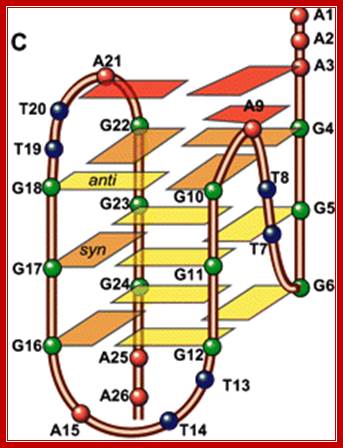
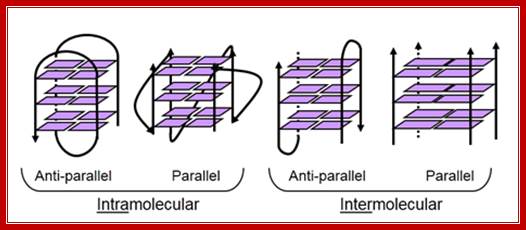
G-Quadruples DNA is highly polymorphic, varying in the 5 to 3’ orientation of the backbone; the orientation of the loops and the number of strands involved. http://www.cmri.org.au/
Quadruplex arrangements
Aged cells have been found to have shorter telomeric segments.
· But proliferating cells have long stretches of telomeric sequences.
Patient who suffers from Progeria disease, what is called as premature aging, have very short telomeric DNA.
· It is difficult to say, whether premature aging causes telomere deletion or telomere deletion cause aging.
It looks aging causes telomere deletions?.
· Conflict- longer life-keep telomeres longer; and don’t say I got cancer.
Development of Novel Human Telomere-Targeted Approaches Yan XU (Assistant Professor), Kunihiro KAWATSU (M2);
The telomeric overhang DNA is also a substrate for telomerase, which elongates telomeric sequence by adding G-rich repeats. Telomerase is activated in 80-90% of human tumors and is low or undetectable in most normal somatic cells. Thus, telomerase or its telomere DNA substrate presents a target with good selectivity for tumor over healthy tissue. Recently, we developed a structure-based approach to sequence-specific cleaving of human telomeric DNA by G-quadruplex formation.

http://www.ch.ic.ac.uk/
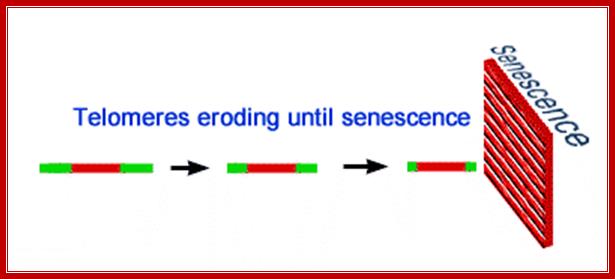
The telomere protects the ends of chromosomes keeping them from recombining with each other. It also serves as a buffer between the requisite genes in each chromosome and the natural erosion of chromosome ends that occurs with each round of DNA replication. Of them from recombining with each other. It also serves as a buffer between the requisite genes in each chromosome and the natural erosion of chromosome ends that occurs with each round. http://www.ch.ic.ac.uk/


Schematic Representations of the composition of telomeric complexes of (a) the yeast S. cerevisiae telomere and (b) the human telomere. Telomeric and nontelomeric DNA are represented as red and grey tubes, respectively. Histone octamers are depicted as orange cylinders. Other components of the telomere complex are labeled. R.A. McCorda, D. Broccolib Schematic representation of telomere structure. Telomeres are at the extremities of chromosome DNA. The telomeric 3′ end terminates as a single-stranded, G-rich overhang able to form the t-loop, in which the overhang invades the telomeric double helix, remodeling the DNA into a circle. Telomeres are capped by at least 6 proteins (TRF1, TRF2, TPP1, POT1, TIN2, and Rap1), collectively known as shelterin, that physically shield the DNA.19 TRF1, TRF2, and TPP1 specifically recognize and bind to double-stranded TTAGGG repeats; POT1 binds to the single-stranded telomeric overhang19,20; TIN2 and Rap1 complete the shelterin complex. Shelterin allows discrimination of telomeres from double-stranded DNA breaks; lack of shelterin allows telomeres to be identified as double-stranded DNA breaks and triggers DNA-damage pathways. http://www.bloodjournal.org

Mutations in telomerase complex genes and human disease. Mutations in green were described in patients with acquired aplastic anemia; mutations in red were described in patients with dyskeratosis congenita; mutations in black were described in patients with pulmonary fibrosis; and polymorphisms are represented in blue. Mutations found in more than one disease type are double-colored. http://www.bloodjournal.org
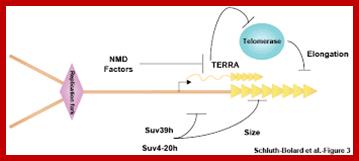
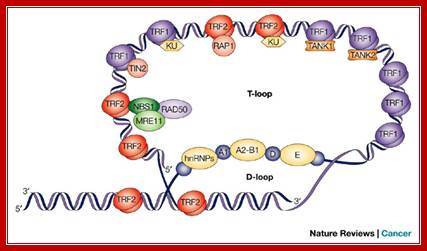
Telomeric end’s maintenance;http://www.nature.com/

Telomeric DNA is bound by a number of different proteins which build up a protective cap on telomeric end; some of the proteins involved are shown below; http://www4.lu.se/ DNA at Telomeric ends is associated with several proteins making the DNA or any genes present repressed. Telomeric DNA is associated with RAP1 proteins (related to Ras associated proteins). The Ras proteins are bound by Sir Proteins such as Sir 2, Sir, 3 and Sir4; Sir stands for Silent information regulators (Silencing regulator proteins); one of the Sir proteins i.e. Sir 2 is a histone deacetylase. Telomeric DNA ends associated RAP1 bound proteins are bound by Rif (Rap integration factor) which is associated with Sir proteins folds on its back to interact with Sir proteins that makes the region totally inactive in terms of gene activity. Sir proteins are also involved in gene silencing by heterochromatization at other regions of the genome. http://www4.lu.se/
Toru M Nakamura, Ph.D.
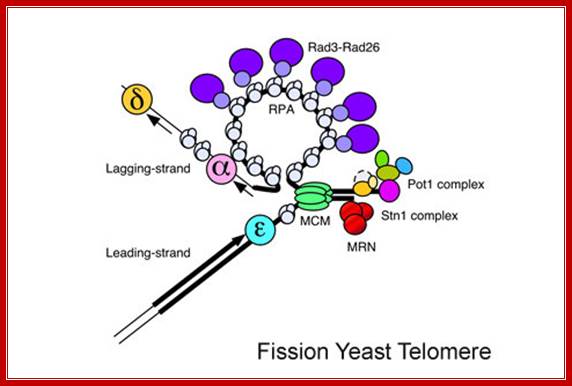
“Our laboratory (i.e. Nakumara’s) is interested in understanding how checkpoint and DNA repair proteins contribute to maintenance of telomeres, the natural ends of linear eukaryotic chromosomes.”
We use fission yeast Schizosaccharomyces pombe as a model system. We have recently demonstrated by quantitative chromatin immunoprecipitation assays that the leading strand DNA polymerase (Pol ε) arrives to replicating telomeres significantly earlier than the lagging strand DNA polymerases (Pol α and Pol δ), and replicating telomeres strongly recruit Replication Protein A (RPA) and Rad3-Rad26 (ATR-ATRIP) complexes in fission yeast. Furthermore, we have also established the cell-cycle-regulated recruitment timing for MCM, Mre11-Rad50-Nbs1 (MRN) complex, Trt1 (TERT, catalytic subunit of telomerase), and telomere capping proteins (Pot1 and Stn1). In another study, we have established that Tel1 (ATM) and Rad3 (ATR) are redundantly required to promote telomere protection and telomerase recruitment by promoting efficient recruitment of the telomere capping complex subunit Ccq1 to telomeres. Finally, we have recently uncovered a surprising kinase-independent role for Rad3 (ATR) kinase in promoting recruitment of Tel1 (ATM) to telomeres in fission yeast. This role of Rad3 (ATR) appears to function redundantly with an alternative Nbs1-dependent Tel1 (ATM) recruitment mechanism, which requires an evolutionarily conserved C-terminal Tel1/ATM-interaction domain of Nbs1. We have further found that the N-terminus of Nbs1 also contributes to recruitment of the Rad3-Rad26 (ATR-ATRIP) complex to telomeres.[ Pot1- protection of Telomere Protein1]. This protein functions as a member of a multi-protein complex that binds to the TTAGGG repeats of telomeres, regulating telomere length and protecting chromosome ends from illegitimate recombination, catastrophic chromosome instability, and abnormal chromosome segregation. Pot1 proteins specifically bind the single stranded overhang at the ends of telomeric DNA. Mol.wt 56.686? Toru M. Nokumara; http://www.uic.edu/
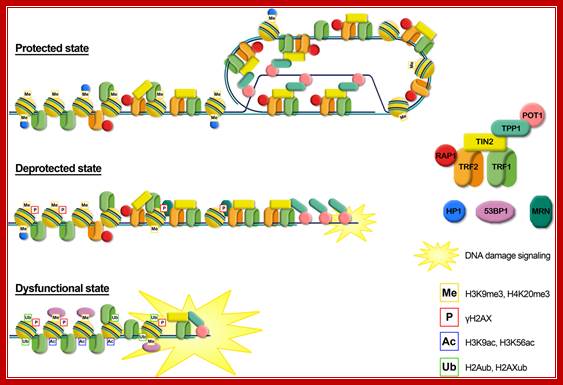
Graphical representation of the different telomere states, characterized by different levels of telomeric proteins and post-translational modifications. Protected state: telomere is in a closed form, probably the t-loop, maintained by the binding with the shelterin proteins; the presence of trimethylation of histones H3 and H4, typical heterochromatic markers, induces a compacted state. This state inhibits the DNA damage response. Deprotected state: telomere shortening could disrupt the closed structure leading to an open state, characterized by a decrease of heterochromatic marks. Telomeres are recognized as DNA damage, signaled by phosphorylation of H2AX, but retain enough shelterin proteins (mainly TRF2) to prevent NHEJ and thus telomeric fusion. DNA damage signaling leads to replicative senescence. Dysfunctional state: if growth arrest checkpoint is inactivated, telomeres continue to shorten leading to a fully uncapped form, deriving from the depletion of shelterin proteins such as TRF2 or POT1. Telomere dysfunctions are signaled by phosphorylation of H2AX and the ubiquitylation of H2A and H2AX. Telomeres are not protected from the DNA damage response machinery, giving rise to extensive telomere fusions. Alessandra Galati et al;http://www.frontiersin.org/

Replication transiently generates positive supercoiling ahead of the elongating fork, which is usually rapidly relaxed by topoisomerases. However, the lock at the basis of the t-loop, which is thought to be formed by a complex nucleoprotein architecture involving a D-loop and a four-way junction, is unlikely to be free to rotate and can be considered as a topological barrier. Therefore, when the fork approaches a t-loop, one expects an accumulation of positive supercoiling in the unreplicated DNA in front of the t-loop and, ultimately, fork pause or arrest. This blockade could be rapidly relieved by t-loop opening and progression of the fork towards the very end of the chromosome (central part of the figure). It could also be efficiently released by topoisomerases and t-loop-opening activities (right part of the figure). Alternatively, if the action of topoisomerases is uncompleted in the presence of a t-loop, a residual positive supercoiling might favour fork regression and the formation of a four-way junction, called a chicken foot (left part of the figure). Chicken-foot regression or resolution, together with t-loop opening, will rescue the blocked fork. It is noteworthy that TTAGGG-repeat factor-2 (TRF2) specifically binds several of these DNA structures, perhaps as part of different shelterin subcomplexes. This might reverse the chicken foot and t-loop through TRF2-dependent activation of processes that are known to disrupt these structures (see Box 2 and main text). Positive supercoils are also expected to represent a favoured substrate for TRF2 binding (see main text), possibly leading to a high concentration of TRF2 ahead of the fork, which might promote t-loop opening. http;//www.nature.com
Telomeric chromatin has been generally considered as “heterochromatic,” mainly on the basis of extensive studies on yeast and Drosophila telomeres, in which the establishment of a heterochromatic state at telomeres and subtelomeres is essential for the protection of chromosome ends (Shore, 2001; Raffa et al., 2011). In budding yeast telomeres are short and form a nucleosome-free structure (Wright et al., 1992). Telomeric double-stranded repeats are bound by the protein RAP1 which recruits among other proteins the Sir complex [Silent Information Regulators, Sir2 (a histone deacetylase), Sir3, and Sir4]. The Sir complex is essential for the formation of a heterochromatic complex that spreads in the subtelomeric region, giving rise to the repression of nearby genes. human CD4+ T-cells showed that telomeres are significantly enriched in H2BK5me1 and H3K4me3, two post-translational histone modifications often found associated with actively transcribed genes. http://journal.frontiersin.org/
