Signal Transduction V-
Protein Kinases and Phosphatases
Protein Kinases:
PKB:
Protein kinases PKBs get activated by cAMP. The PKB enzymes play important roles. The Phosphotidyl Inosine phosphatePI3 that is released targets PKB, which is a serine/threonine kinase. Its catalytic domain and PH domain binds to phosphate groups of both Pi3’4 and Pi3’4’5-triphosphates. Binding of PI3 to PKB localizes PKB at membrane surface. The PH domain i.e. phosphate binding domain conceals active/catalytic domain; the binding of phosphate opens up the catalytic domain. Fully activated PKB dissociates from the membrane.
In many types of cells, stress and irreparable damage to cells lead to apoptosis. But in some case PKB prevent apoptosis by p-lating pro-apoptotic proteins, ex.BAD an apoptotic factor. PKB also p-lates several proteins including some TFs such as Forkhead1 at several ser residues. Fork head protein not p-lated remains in the nucleus and activates several genes encoding pro-apoptosis. This happens when cells are not stimulated for growth, but when stimulated PKB gets activated and fork head protein gets p-lated. This makes phosphor serine binding protein 14.3.3 bind to forkhead1 and sequesters in cytosol, thus pro-apoptotic genes are inactivated. The 14.3.3 protein also retains Raf in cytosol. Inactivation of PKB leads to dephosphorylation of fork head, thus it enters the nucleus and activates proapoptotic genes.
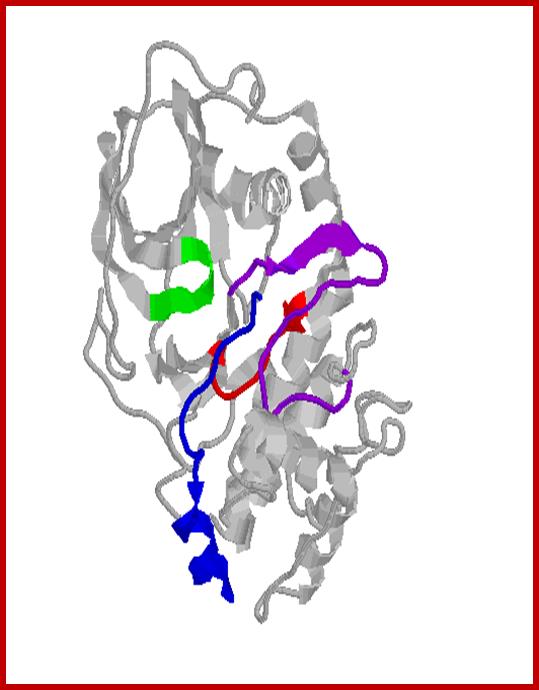
Protein kinase-A in active conformation; http://www.qub.ac.uk/
Cyclic AMP is an important second messenger. It forms, as shown, when the membrane enzyme adenylyl cyclase is activated (as indicated, by the alpha subunit of a G protein).
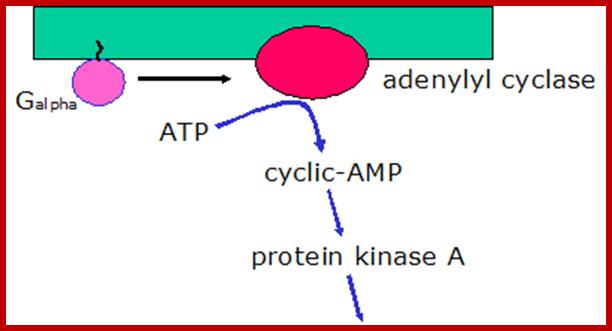
The cyclic AMP then goes on the activate specific proteins. Some ion channels, for example, cyclic AMP induces gated ion channels. But an especially important protein activated by cyclic AMP is protein kinase A, which goes on the phosphorylate certain cellular proteins. The scheme below shows how cyclic AMP activates protein kinase A.

Activation of Protein kinase-A; http://courses.washington.edu/

Structure of kinase domain of PKCβII showing priming site phosphorylations: activation loop (pink), turn motif (orange), and hydrophobic motif (green)). Also shown is the clamp between the PXXP motif (Pro, in green) and the conserved Tyr (yellow) of the αE helix; Protein kinase-C; http://ajpendo.physiology.org/
Phosphatases:
As kinases are important for the health of cells, phosphotases are also important for they counteract each other. There are three kinds of kinases, receptor protein kinases, cellular protein kinases and there are non protein kinases which p-lates several kinds of substrates such as carbohydrates, lipid and other non protein components, most of them ATP dependent.
Many proteins are activated by kinases and the same are inactivated by specific phosphatases that removes phosphates from tyrosine, threonine, serine or histidine. Intricate play between protein kinases or non protein kinases and phosphotases is critical for the health of the cell. Similarly there are many proteins get inactivated by kinase p-lation and there are proteins get activated by the removal phosphates by phosphotases.
PTEN is a phosphatase which has broad range of targets. It can remove phosphorus from ser. threonine and tyrosine. But removal of phosphorus from PI3 is significant.
![Phosphatases in cell|[ndash]|matrix adhesion and migration](Cellular_Signal_Transduction5-Protein_Kinases_And_Phosphotases_files/image005.jpg)
The PTP family includes soluble PTPs, receptor PTPs (RPTPs), dual-specificity PTPs (DUSPs) and low-molecular-weight PTPs (LMW-PTPs). The soluble PTPs and RPTPs have diverse structures, yet their catalytic domains are remarkably well conserved and have nearly identical three-dimensional configurations. The PTPs bind only to the phosphorylated forms of their substrates, and the binding site recognizes the phosphorylated tyrosine and adjacent residues. Therefore, the catalytic domains, other localization motifs and protein–protein-interaction domains that are present on phosphatases primarily dictate the high selectivity, in vivo, of this class of phosphatases (reviewed in Ref. 115). The PTP catalytic domain is highly conserved — and contains a single cysteine that is used in a cysteinyl-phosphate enzyme intermediate during dephosphorylation. An extended description of the PTP family can be found on the phosphatases web site in the Online links. Melinda Larsen, Michel L. Tremblay & Kenneth M. Yamada; http://www.nature.com/
The DUSPs can dephosphorylate phosphorylated tyrosine, threonine and serine residues, although specificity differs among individual phosphatases. DUSPs also share a high degree of homology in their catalytic domains, but do not depend on the recognition of the phosphorylated residue for substrate binding — instead; they bind through a separate binding domain114. Docking interactions are therefore essential for regulating the specificity of the DUSPs, although the mechanisms have only recently become understood. Phosphatase and tensin homologue (PTEN), which is a phosphatidylinositol 3,4,5-trisphosphate lipid phosphatase, as well as a tyrosine phosphatase, can sometimes be classified as a DUSP.
Phosphatases have diverse structures:
Protein phosphatases can be classified as protein tyrosine phosphatases (PTPs) or serine/threonine phosphatases (PPs). The PPs dephosphorylate phosphoserines and phosphothreonines and are completely different in structure from the PTPs in that they are holoenzymes. PPs are composed of a catalytic subunit that binds to either one or two other regulatory subunits, which can be inhibitory. For clarity, in this figure and others, we have depicted the PPs as a single subunit, although they are always multiple-subunit proteins. There are two subfamilies of PPs: the PPP family, which includes PP1-, PP2A-, PP2B- and PP5-type phosphatases; and the PPM family, comprising PP2C-type and mitochondrial phosphatases. PP catalytic domains are highly conserved, have similar three-dimensional structures and use similar catalytic mechanisms. PPs, however, dephosphorylate their substrates using a metal-activated, nucleophilic, water molecule in a single reaction step116. Also mentioned in this review are the Src-homology-2 (SH2)-domain-containing inositol 5'-phosphatases (SHIPs). They are not protein phosphatases and are, at present, classified in the endonuclease/exonuclease/phosphatase family (see SHIPs in the Online links)117. DEP1, high cell-density enhanced PTP-1; JSP1, Jun amino-terminal kinase (JNK) stimulatory phosphatase; LAR, leukocyte common antigen related; MLCP, myosin light-chain phosphatase; POPX1/2, partner of PIX (PAK-interacting exchange factor) 1/2; PTP-PEST, PTP-proline, glutamate, serine and threonine-rich domain; SAP1, stomach cancer-associated PTP; SHP, SH2-domain-containing PTP. The DUSPs can dephosphorylate phosphorylated tyrosine, threonine and serine residues, although specificity differs among individual phosphatases. DUSPs also share a high degree of homology in their catalytic domains, but do not depend on the recognition of the phosphorylated residue for substrate binding — instead, they bind through a separate binding domain114. Docking interactions are therefore essential for regulating the specificity of the DUSPs, although the mechanisms have only recently become understood. Phosphatase and tensin homologue (PTEN), which is a phosphatidylinositol 3,4,5-trisphosphate lipid phosphatase, as well as a tyrosine phosphatase, can sometimes be classified as a DUSP.
The PPs dephosphorylate phosphoserines and phosphothreonines and are completely different in structure from the PTPs in that they are holoenzymes. PPs are composed of a catalytic subunit that binds to either one or two other regulatory subunits, which can be inhibitory. For clarity, in this figure and others, we have depicted the PPs as a single subunit, although they are always multiple-subunit proteins. There are two subfamilies of PPs: the PPP family, which includes PP1-, PP2A-, PP2B- and PP5-type phosphatases; and the PPM family, comprising PP2C-type and mitochondrial phosphatases. PP catalytic domains are highly conserved, have similar three-dimensional structures and use similar catalytic mechanisms. PPs, however, dephosphorylate their substrates using a metal-activated, nucleophilic, water molecule in a single reaction step116. Also mentioned in this review are the Src-homology-2 (SH2)-domain-containing inositol 5'-phosphatases (SHIPs). They are not protein phosphatases and are, at present, classified in the endonuclease/exonuclease/phosphatase family (see SHIPs in the Online links)117. DEP1, high cell-density enhanced PTP-1; JSP1, Jun amino-terminal kinase (JNK) stimulatory phosphatase; LAR, leukocyte common antigen related; MLCP, myosin light-chain phosphatase; POPX1/2, partner of PIX (PAK-interacting exchange factor) 1/2; PTP-PEST, PTP-proline, glutamate, serine and threonine-rich domain; SAP1, stomach cancer-associated PTP; SHP, SH2-domain-containing PTP. Melinda Larsen et al>

Signaling through p38alphaMAPK. Signaling through MAPK14 cascade and its role in the regulation of cellular functions; MAPK14 is involved in signaling pathways triggered by a variety of stimuli such as growth factors, oxidative stress, UV, cytokines and DNA damage. Depending on the stimulus, different receptors and intermediates (adaptors, GTPases or kinases) are activated leading to the activation of the p38alpha MAPK cascade. This cascade is initiated by activation of MAPKKKs, which phosphorylate and activate MAPKKs (MKK3/6/4), which in turn lead to activation of MAPK14 through dual phosphorylation in Tyr and Thr. Once phosphorylated, MAPK14 phosphorylates a number of cytosolic and nuclear substrates, including transcription factors, which lead to the control of many cellular responses. http://atlasgeneticsoncology.org/
p38alpha is mainly activated by various environmental stresses and proinflammatory cytokines, but many other extracellular signals, including growth factors, also lead to p38alpha activation. The canonical activation requires its phosphorylation in threonine and tyrosine residues by dual-specificity MAP kinase kinases (MKKs), MKK3, MKK6 and MKK4. Substrates of this kinase include transcription factors and other proteins.
Phosphatase and tensin homolog (PTEN): http://en.wikipedia.org/
Over expression PTEN in cells by recombinant methods leads to cell apoptosis, this is due to the removal phosphate groups from PI3. PTEN is often deleted from several cancer cells. Its’ loss leads to uncontrolled growth of cells. Lack of PTEN components lead to increased levels of PI 3, 4, 5 and PKB activities. Since PKB exerts anti apoptotic activity; PTEN indirectly reduces apoptotic of abnormal cells; this leads to the stimulation cell cycle and proliferation.


Phosphorylation is a reversible PTM that regulates protein function. Left panel: Protein kinases mediate phosphorylation at serine, threonine and tyrosine side chains, and phosphatases reverse protein phosphorylation by hydrolyzing the phosphate group. Right panel: Phosphorylation causes conformational changes in proteins that either activate (top) or inactivate (bottom) protein function. http://www.piercenet.com/
Phosphatases and epithelial–Mesenchymal transition:
Phosphatases, in addition to regulating cell–matrix adhesion, also
modulate cell–cell adhesions. Epithelial cells are present in vivo as sheets of cells that are connected
by cell–cell adhesions, such as ADHERENS JUNCTIONS, that
must be broken down prior to migration. Phosphatases participate in the
regulation of adherens junctions, but this is just one of their many functions
in cell–cell adhesion. During EPITHELIAL–MESENCHYMAL
TRANSITION (EMT), E-cadherin in adherens junctions becomes
phosphorylated. This releases the ![]() -catenin that was bound
to the cytoplasmic tail of E-cadherin, into the cytoplasm. Expression of
PTP-LAR (protein tyrosine phosphatase-leukocyte antigen-related) inhibits
E-cadherin phosphorylation and favors epithelial integrity
-catenin that was bound
to the cytoplasmic tail of E-cadherin, into the cytoplasm. Expression of
PTP-LAR (protein tyrosine phosphatase-leukocyte antigen-related) inhibits
E-cadherin phosphorylation and favors epithelial integrity
![Phosphatases in cell|[ndash]|matrix adhesion and migration](Cellular_Signal_Transduction5-Protein_Kinases_And_Phosphotases_files/image011.jpg)
Phosphatases in cell–matrix adhesion and
migration;The
serine/threonine protein phosphatase, PP2A, has a complex relationship with ![]() -catenin and
signalling by the Wnt ligand and the Frizzled receptor (Fz). PP2AC
-catenin and
signalling by the Wnt ligand and the Frizzled receptor (Fz). PP2AC![]() — which is
composed of the C
— which is
composed of the C![]() and A
subunits — associates with E-cadherin and catenin complexes and actively
maintains E-cadherin–
and A
subunits — associates with E-cadherin and catenin complexes and actively
maintains E-cadherin–![]() -catenin interactions.
The phenotype of PP2AC
-catenin interactions.
The phenotype of PP2AC![]() -null mice
— which die at embryonic day 6.5 and lack mesoderm90 — is apparently due to cytoplasmic localization
and subsequent degradation of phosphorylated
-null mice
— which die at embryonic day 6.5 and lack mesoderm90 — is apparently due to cytoplasmic localization
and subsequent degradation of phosphorylated ![]() -catenin. However, if
-catenin. However, if ![]() -catenin is released
from the E-cadherin cytoplasmic tail, PP2A participates in its activation. A
holoenzyme form of PP2A, which consists of the A, C
-catenin is released
from the E-cadherin cytoplasmic tail, PP2A participates in its activation. A
holoenzyme form of PP2A, which consists of the A, C![]() , and B' subunits
(PP2AC
, and B' subunits
(PP2AC![]() ), is a
component of a cytoplasmic complex that contains
), is a
component of a cytoplasmic complex that contains ![]() -catenin, the
adenomatous polyposis coli protein (APC), glycogen-synthase kinase-3
-catenin, the
adenomatous polyposis coli protein (APC), glycogen-synthase kinase-3![]() (GSK-3
(GSK-3![]() ) and axin122. When PP2A is held in an inactive state by the B' subunit, GSK-3
) and axin122. When PP2A is held in an inactive state by the B' subunit, GSK-3![]() kinase
activity predominates,
kinase
activity predominates, ![]() -catenin is
phosphorylated, becomes ubiquitylated and is targeted for degradation123 (left figure). In response to a Wnt/Frizzled
signal, GSK-3
-catenin is
phosphorylated, becomes ubiquitylated and is targeted for degradation123 (left figure). In response to a Wnt/Frizzled
signal, GSK-3![]() activity is inhibited
by Dishevelled (dsh) phosphorylation, and PP2A is activated (indicated by an
asterisk). PP2A then apparently dephosphorylates
activity is inhibited
by Dishevelled (dsh) phosphorylation, and PP2A is activated (indicated by an
asterisk). PP2A then apparently dephosphorylates ![]() -catenin, which is
stabilized and transported into the nucleus, where it can participate with
Lef/TCF (lymphoid enhancer binding factor/T-cell factor) in the transcriptional
induction of genes that are required for EMT124 (right figure). Interestingly, another
phosphatase has recently been reported to oppose the effects of PP2A. By an
indirect and as yet unknown mechanism, PTEN (phosphatase and tensin homologue)
promotes GSK-3
-catenin, which is
stabilized and transported into the nucleus, where it can participate with
Lef/TCF (lymphoid enhancer binding factor/T-cell factor) in the transcriptional
induction of genes that are required for EMT124 (right figure). Interestingly, another
phosphatase has recently been reported to oppose the effects of PP2A. By an
indirect and as yet unknown mechanism, PTEN (phosphatase and tensin homologue)
promotes GSK-3![]() activation
and subsequent degradation of
activation
and subsequent degradation of ![]() -catenin125. This signalling pathway, controlling the activation/degradation of
-catenin125. This signalling pathway, controlling the activation/degradation of ![]() -catenin, is an
example in which a single phosphatase has several roles, and in which a
phosphatase is involved in both activation and inhibition of signalling. Melinda
Larsen, Michel L. Tremblay & Kenneth M. Yamada; http://www.nature.com/
-catenin, is an
example in which a single phosphatase has several roles, and in which a
phosphatase is involved in both activation and inhibition of signalling. Melinda
Larsen, Michel L. Tremblay & Kenneth M. Yamada; http://www.nature.com/

a | Receptor tyrosine
kinase (RTK) ligands bind to the extracellular domain of RTKs. This leads to
dimerization of two receptor subunits and intracellular trans-autophosphorylation
on tyrosine residues. Inhibition of receptor-directed protein-tyrosine
phosphatases (PTPs) is another important aspect of receptor activation.
Signalling molecules then bind to the phosphotyrosine sites, which leads to
their activation. One important signalling pathway is indicated: the adaptor
molecule Grb2 binds directly or
indirectly through another adaptor, Shc, and recruits Sos to the receptor,
which loads GTP onto Ras. Ras then activates the ERK/MAPK cascade — through Raf
and MEK — which is linked to activation of cell proliferation. b | G-protein-coupled receptors (GPCRs)
have a wide range of relatively small, structurally diverse ligands.
Extracellular ligand binding to GPCRs leads to GTP-loading of the G![]() -subunit and dissociation
of the trimeric G-protein complex from the receptor. G
-subunit and dissociation
of the trimeric G-protein complex from the receptor. G![]() and G
and G![]()
![]() subunits elicit
intracellular signals through protein–protein interactions. Many GPCRs can also
activate the conserved ERK/MAPK pathway and stimulate cell proliferation in
some cell types. Active and inactive states are indicated by 'traffic light'
coding — green and red, respectively. ERK, extracellular signal-regulated
kinase; Grb2, growth factor receptor-bound 2; MAPK, mitogen-activated protein
kinase; MEK, MAPK and ERK kinase; Shc, Src-homology-2-containing; Sos,
son-of-sevenless; TM, transmembrane.
http://www.nature.com/
subunits elicit
intracellular signals through protein–protein interactions. Many GPCRs can also
activate the conserved ERK/MAPK pathway and stimulate cell proliferation in
some cell types. Active and inactive states are indicated by 'traffic light'
coding — green and red, respectively. ERK, extracellular signal-regulated
kinase; Grb2, growth factor receptor-bound 2; MAPK, mitogen-activated protein
kinase; MEK, MAPK and ERK kinase; Shc, Src-homology-2-containing; Sos,
son-of-sevenless; TM, transmembrane.
http://www.nature.com/
Protein Kinases:
PK-B:
Like PKA activated by cAMP, the PK-B enzymes also play important roles. The PI-3 that is released targets PKB, which is a serine/threonine kinase. It’s catalytic domain and PH domain binds to phosphate groups of both Pi3’4 and Pi3’4’5-triphosphates. Binding of PI3 to PKB localizes PKB at membrane surface. The PH domain i.e. phosphate binding domain conceals active/catalytic domain; the binding of phosphate opens up the catalytic domain. Fully activated PKB dissociates from the membrane.
In many types of cells, stress and irreparable damage to cells lead to apoptosis. But in some case PKB prevent apoptosis by p-lating pro-apoptotic proteins, ex.BAD. PK-B also p-lates several proteins including some TFs such as Forkhead1 at several ser residues. Fork head protein not p-lates remains in the nucleus and activates several genes encoding proapoptosis. This happens when cells are not stimulated for growth, but when stimulated PKB gets activated and fork head protein gets p-lated. This makes phosphor serine binding protein 14.3.3 bind to forkhead1 and sequesters in cytosol, thus pro-apoptotic genes are inactivated. The 14.3.3 protein also retains Raf in cytosol. In activation of PKB leads to dephosphorylation of fork head, thus it enters the nucleus and activates proapoptotic genes.
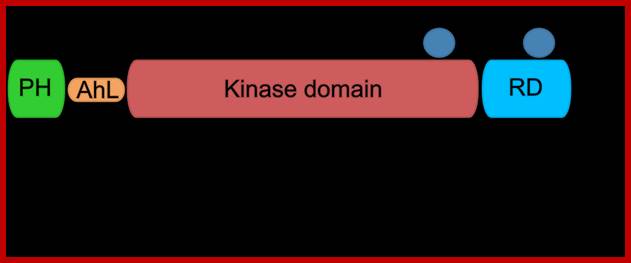
Protein kinase-B domains; http://www.actuscimed.com/
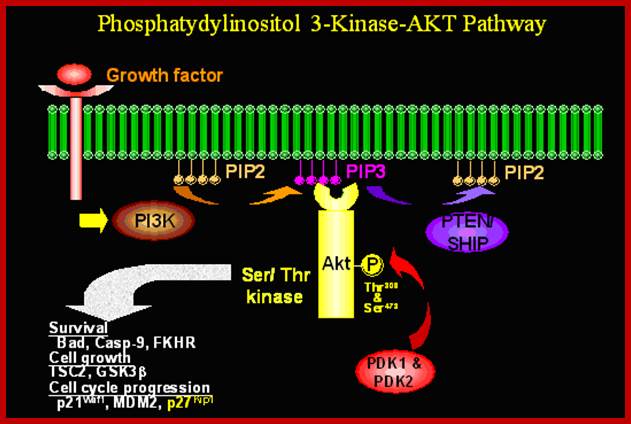
PI3 Kinase; ;Activation of protein kinase-B; http://werner.yellowcouch.org/
Phosphatases:
As kinases are important for the health of cells, phosphotases are also important for they counteract each other. There are three kinds of kinases, receptor protein kinases, cellular protein kinases and there are non protein kinases which p-lates several kinds of substrates such as carbohydrates, lipid and other non protein components, most of them ATP dependent.

Kinases and phosphatases typically counter each other's functions in regulating phosphorylation of their common substrates. Kinases and phosphatases can also target themselves or each other - phosphatases can dephosphorylate kinases, thus affecting their properties (down-regulating or increasing catalytic activity, adding or removing binding sites of other proteins, etc.). Phosphatases can also auto-dephosphorylate, thereby altering their own properties. ;http://www.weizmann.ac.il
Many proteins are activated by kinases and the same are inactivated by specific phosphatases that removes phosphates from tyrosine, threonine, serine or histidine. Intricate play between protein kinases or non protein kinases and phosphotases is critical for the health of the cell. Similarly there are many proteins get inactivated by kinase p-lation and there are proteins get activated by the removal phosphates by phosphotases. PTEN is a phosphotase which has broad range of targets. It can remove Phosphorus from ser. threonine and tyrosine. But removal of phosphorus from PI3 is significant.
Over expression PTEN in cells by recombinant methods leads to cell apoptosis, this is due to the removal phosphate groups from PI3. PTEN is often deleted from several cancer cells. Its’ loss leads t uncontrolled growth of cells. Lack of PTEN molecules lead to increased levels of PI3, 4, 5 and PKB active. Since PKB exerts anti apoptotic activity, PTEN indirectly reduces apoptotic of abnormal cells; this leads to the stimulation cell cycle and proliferation.
Tyrosine phosphatases:
When PTEN gene is knocked out of a mouse, the brain of mice grows larger in size and with excess neurons; this is the profound effect of PTEN on brain.

For a review on the PTPs see: Tiganis, T., and Bennett, A.M. (2007) Protein tyrosine phosphatase function: the substrate perspective. Biochem J. 402, 1-15; http://www.med.monash.edu.au/
?
Down regulation signal induced activities:
The levels of hormones produced and released from signaling cells are adjusted to the needs of an organism. When oxygen level is low kidney cells produce more erythropoietin and release. Cellular Ski and Sos ate induced following TGFb and cytokine action, which negatively regulate their respective signaling pathway. P-lation of receptors and downstream signaling proteins are reversed by specific phosphotases. Another mode of control is to remove receptors from the surface. Another mode is to sequester signaling molecules till required. In the case of LDL receptors are efficiently recycled but several of them are transferred to lysosomes where both ligand and receptors are degraded. Ex. EGF-R is internalized when EGF bind to the receptors, nearly 50% of them are degraded. Exposure of fibroblasts to high level of EGF for an hour or more leads to several rounds of endocytosis, thus reduction in the number of receptors. When the concentration of exoplasmic EGF is reduced EGF receptors are restored to their original numbers. It is also possible by dephosphorylation of receptors. Thus cells can be sensitized and desensitized. In the case of IL2 that stimulates T-cells, when its concentration is reduced by internalization of both receptors and ligands, end in signaling
Endocytosis mode:
In the case high concentration and persistence signaling molecules for several hours, cells become desensitized, so they don’t respond to further increase in signaling molecules. Endocytosis of ligand and receptors decrease the number of receptors, this is the principle mode of desensitization against more signal molecules especially against peptide and other hormones. Signaling receptors often go through recycling by endocytosis and exocytosis.

http://circabook.com
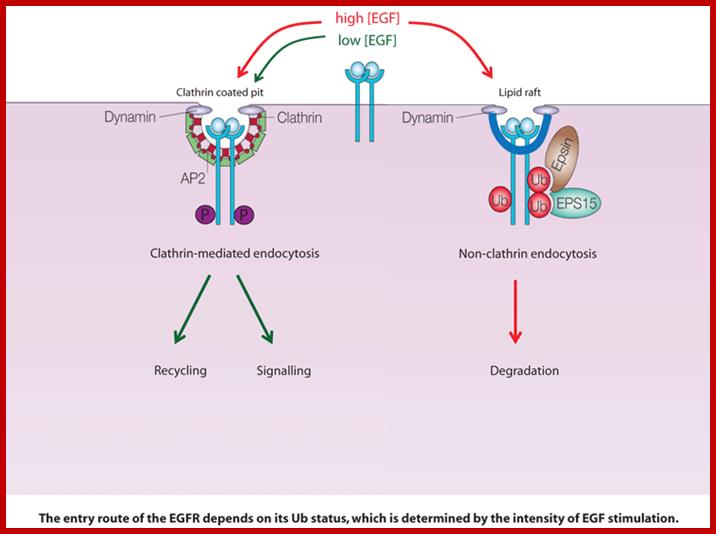
Cell surface receptors can be endocytosed by both clathrin-dependent and clathrin-independent mechanisms, but, as yet, there is no clear understanding of why alternative endocytic routes exist or how receptors are committed to a particular route. We are using the epidermal growth factor receptor (EGFR) as a model 'endocytic system' to address these issues. This kinase receptor has important clinical relevance, as EGFR deregulated signalling is strongly associated with cancer.; http://www.ifom-ieo-campus.it/
Decoy receptors:
Another unique way of reducing signaling activity via receptors is to secrete a protein that binds to signaling molecules and reduce signaling activity. Such h hormone binding molecules are called decoys.
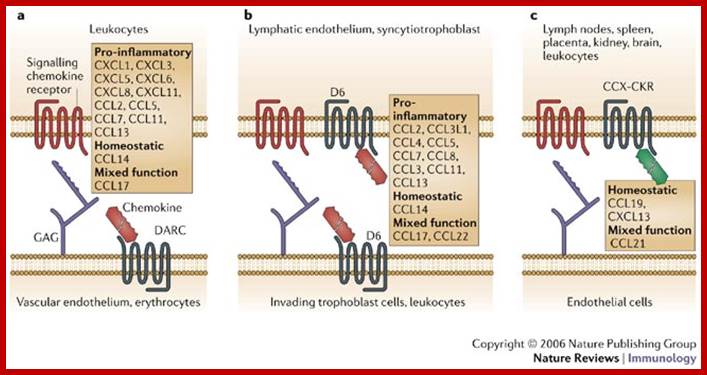
Ligand specificity and tissue distribution of chemokine decoy receptors. Chemokine decoy receptors (in grey) compete with signalling receptors (in red) for interaction with the ligands, thereby preventing cell activation. Ligand specificity and tissue and/or cell-type distribution of individual molecules are shown. DARC (Duffy antigen receptor for chemokines) (a) and D6 (b) are mainly expressed on the endothelium (lymphatic and vascular, respectively); DARC is also expressed by erythrocytes, and some evidence of D6 expression by leukocytes has also been provided. CCX-CKR (c) is expressed by various tissues and by leukocytes, although CCX-CKR-expressing cells have not been definitively identified ;http://www.nature.com/
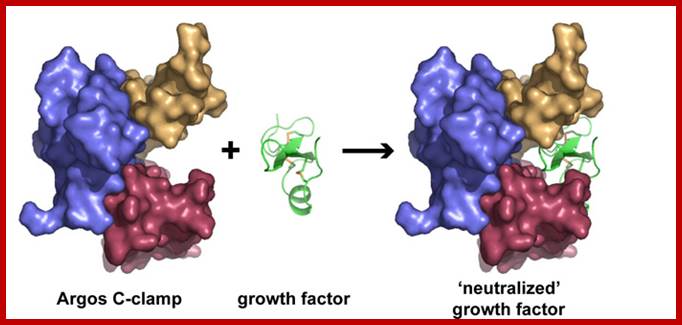
How Argos, a fruit fly protein, acts as a "decoy" receptor,
binding and neutralizing growth factor molecules (green) that promote the
progression of cancer. Credit: Mark Lemmon, PhD, University of Pennsylvania
School of Medicine;
Read more at: http://phys.org/news131210034.html#jCp; http://phys.org/
This type of regulation is found in bone resorption, which is a complex process. Overall growth of bones subsides just before puberty, resorption and bone formation and remodeling is fine tuned in adult hood all the time in their life. Remodeling permits repair of damaged bones and can release calcium phosphate and other ions from mineralized bones into blood for the use of the same elsewhere.
Signal Induced protein cleavage:
Irreversible signal pathway in contrast to reversible pathway is fascinating and also very important. Such pathways lead to degradation of one of the partners of the activated components. NF kB pathway and Notch/delta pathways can be cited as examples. In majority of examples one find signal induced pathway lead to activation of TF via kinase activities. In certain cases signal induced activation of TF is due to the release of TF from inhibitors. A good example is NFkB.
Nuclear factor k B is a master transcriptional regulator of immune system. Especially B-lymphocytes. In fly, Drosophila homologues of NFkB are found; they induce the synthesis of anti microbial peptides. Flies do contain an immune system that differs from higher systems. It is believed that NFkB is an old system existed for more than half billion years. In response to infection with virus, stress factors and inflammation (including ionization radiations) NFkBs are rapidly activated in mammalian immune system. It is also activated by inflammatory cytokines such as tumor necrosis factors (TNFalpha), interleukins (IL-1) and others; they are released b due to infection. P65 and P50 are two subunits of NFkB and they share homology at N-terminal end and it is required for dimerization and binding to specific DNA sequences. In unstimulated cells p65 and p50 are sequestered by another protein called Inhibitor IkB which binds to N-terminal region of dimers. Activation TN alpha ot IL1 receptors specific kinases become active and they p-late IkB at serine site. This releases IkB from the p65-50 dimers and soon IkB ubiquitylated by E3 ligase system, thus polyubiquitinated IkB is fed to proteasome and degraded. Cells mutant to NFkB or mutation of serine to glycine in NFkB subunits make NFkB permanently repressed. Once the dimmers are released from the inhibitor, they move into the nucleus and activate a set of genes. A negative feedback loop operate in this system for one of the NFkB induced gene product is the IkB, which bind to NFkB dimmers and the complex move into cytosol and remain inactivated.
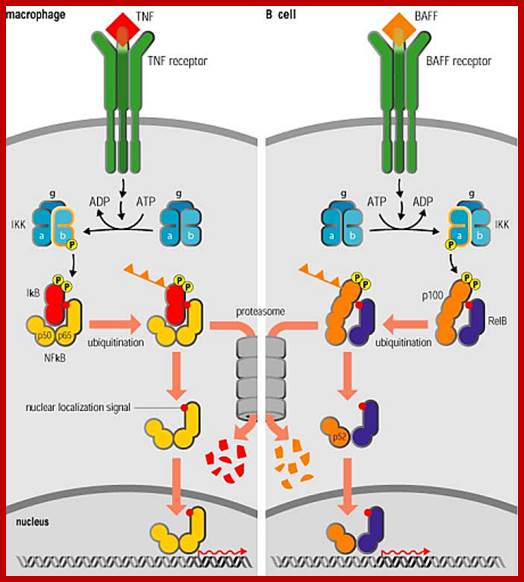
NF-kB activates inflammatory gene expression; Nuclear factor kB (NF-kB) is a transcriptional regulator that plays a central part in responses to inflammatory signaling not only through Toll-like receptors, but also through TNF receptors and the IL-1 receptor, which we discuss next, as well as in the diverse responses to other signals operating through the TNF receptor superfamily (see section 2–7). It is also essential for responses to signalling through the variable antigen receptors of lymphocytes, which we describe later in the book. We shall see later in this section that although the protein is always called NF-kB, it is a group of related homodimeric and heterodimeric transcription factors that are likely to activate distinct sets of target genes. /http://www.stat.rice.edu/
NFkB activates more than 150 genes including those of cytokines and chemokines which attract other immune cells and fibroblast to the site of infection. NFkB also promotes the expression of receptor proteins that enable neutrophils to migrate from blood into underlying tissues. The NFkB also induces the expression of INOs, an inducible isoform of Nitric oxide. Nitric oxide is toxic to bacteria and it is anti -apoptotic proteins. Thus a single species of NFkB protein coordinate and activate bodies’ defense system. NFkB are also involved in developmental aspects. Mice lacking in IFkB die in embryonic stage due to excessive apoptosis. Thus p-Lation dependent degradation of cyclin kinase dependent proteins plays key role in regulating progression of cell cycle. So p-lation dependent protein degradation plays an important role in cellular processes. Notch and delta membrane proteins are also involved in such p-lation induced degradation.