Cellular Signal transduction 3:
Signal Transduction Pathways:
Insulin Receptor activated pathway:
Insulin receptor is a tetrameric proteins with two subunits located in the external domain and two at the cytosolic side of the membranes of fibroblasts, hepatic tissue and muscle cells. Dimeric alpha subunits are linked by S-S bonds and two betas are anchored in the membrane; an unusual set of the receptor proteins. Insulin binding to its receptor leads to dimerization and activation of its tyrosine kinase activity.
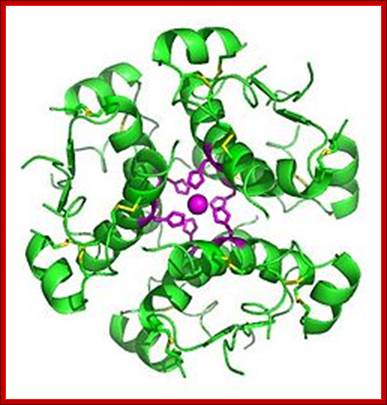
High-resolution model of six insulin molecules assembled in a hexamer, highlighting the threefoldsymmetry, the zinc ion holding it together (pink sphere), and the histidine residues (pink sticks) involved in zinc binding. Inactive insulin is stored in the body as a hexamer, while the active form is the monomer. http://en.wikipedia.org/
Insulin is synthesized in islets of langerhans, beta cells; this pituitary body generates not only insulin, but also glucagon, proteases and G-proteins. Insulin synthesis and release is stimulated by the level of exocellular blood sugar. Insulin is synthesized as pre-pro insulin. During transport into the ER the signal sequences are removed. Then the proinsulin is bundled as proinsulin granules. Enveloped by a membrane and stored in cytoplasm. When proinsulin granules are released proinsulin is processed into active dimeric insulin which is active.
http://www.ghc.org/
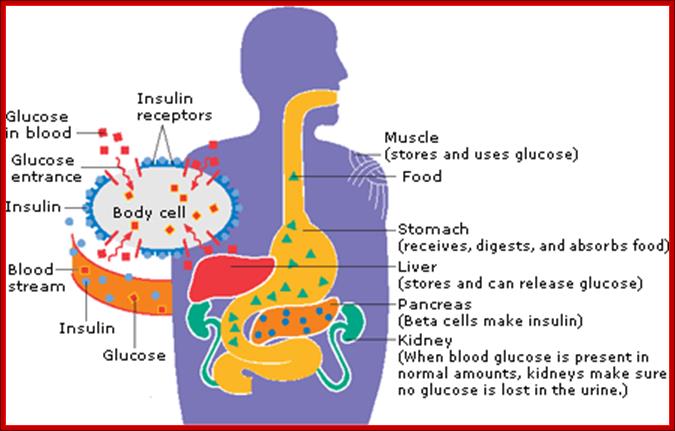
How Insulin works: Insulin is a hormone made by one of the body's organs called the pancreas. Insulin helps your body turn blood sugar (glucose) into energy. It also helps your body store it in your muscles, fat cells, and liver to use later, when your body needs it.
After you eat, your blood sugar (glucose) rises. This rise in glucose triggers your pancreas to release insulin into the bloodstream. Insulin travels through the blood to your body's cells. It tells the cells to open up and let the glucose in. Once inside, the cells convert glucose into energy or store it to use later.
Without insulin, your body can't use or store glucose for energy. Instead, the glucose stays in your blood. http://www.ghc.org/
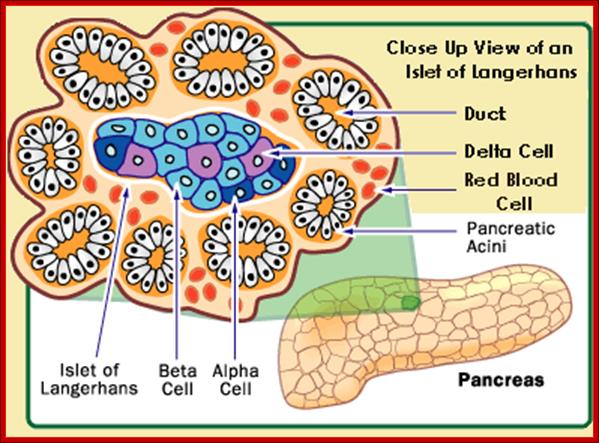
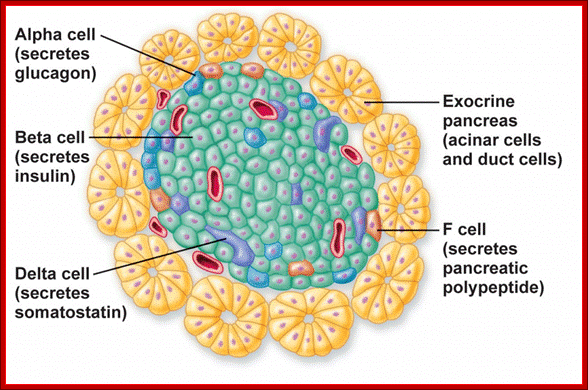
A small group of cells in the pancreas that function as endocrine glands, Alpha cells secret the hormone glucagon, the beta cells secrete Insulin,the delta cells secrete somatostatin, and pancreatic polypeptide cells secrete pancreatic polypeptides; Paul Langerhans discovered the islets now called Islets of langehans; http://teachmeanatomy.info/ and ;http://www.daviddarling.info/
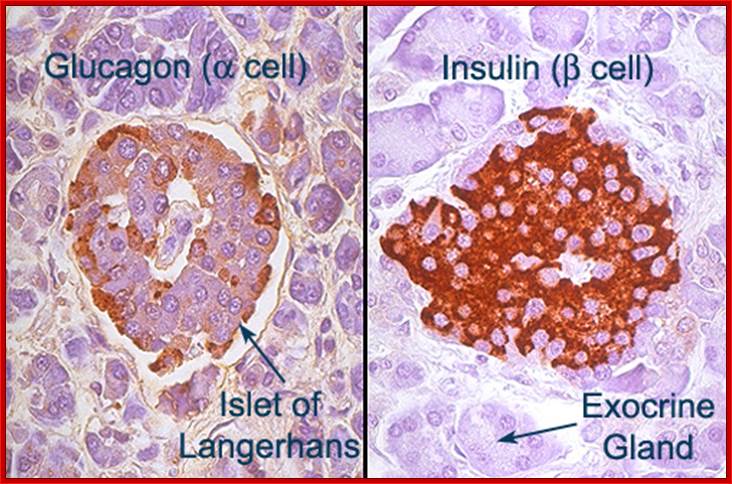
Islets of Langerhans; http://www.daviddarling.info/
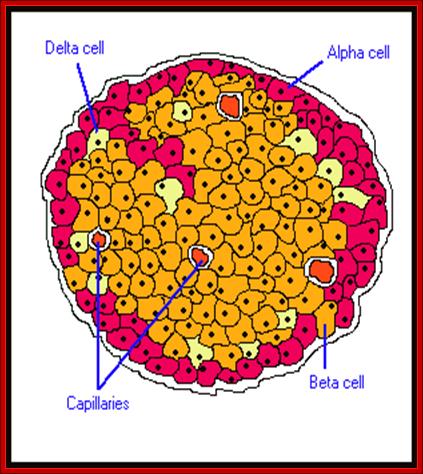
Islets of langerhans is conglomerate of cells consisting of alpha, beta, delta and G cells. It is beta cells that synthesize, store and release insulin. http://www.diabetesindia.com
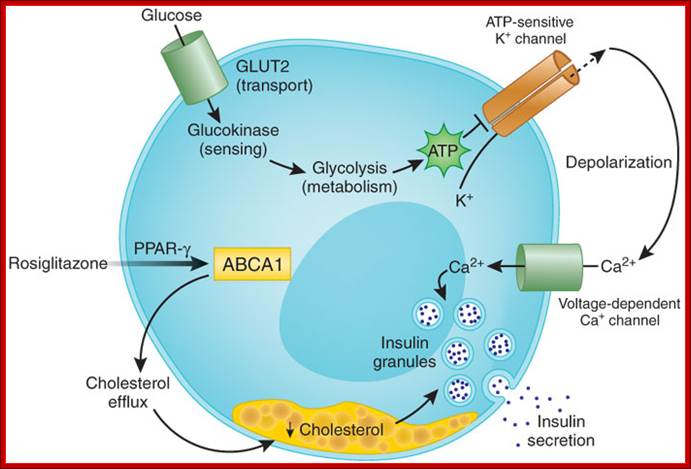
Entry of glucose into cells; Manu V Chakravarthy & Clay F Semenkovich; http://www.nature.com/
The
'classical' pathway involves glucose transport (by means of glucose transporter
2, GLUT2), sensing (by glucokinase) and metabolism (by glycolysis) to generate
ATP, which leads to the closure of ATP-sensitive K+ channels (Kir6.2). This depolarizes
the plasma membrane, opening voltage-dependent Ca2+ channels, which leads to an influx of
extracellular Ca2+ and
thus results in insulin exocytosis. Brunham et
al.2 now
show that ![]() -cell Abca1 is critical
for insulin secretion by modulating cellular cholesterol homeostasis. This
effect is downstream of glucose transport, sensing and metabolism. Increased
insulin secretion mediated by the thiazolidinedione rosiglitazone (which
activates PPAR-
-cell Abca1 is critical
for insulin secretion by modulating cellular cholesterol homeostasis. This
effect is downstream of glucose transport, sensing and metabolism. Increased
insulin secretion mediated by the thiazolidinedione rosiglitazone (which
activates PPAR-![]() ) requires Abca1,
suggesting that the regulation of membrane cholesterol levels affects
) requires Abca1,
suggesting that the regulation of membrane cholesterol levels affects ![]() -cell function in type 2
diabetes.
-cell function in type 2
diabetes.
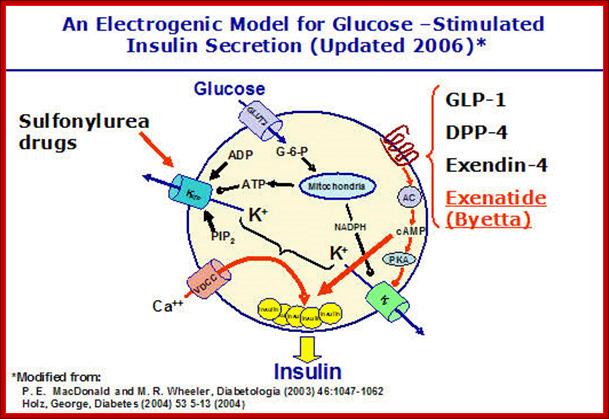
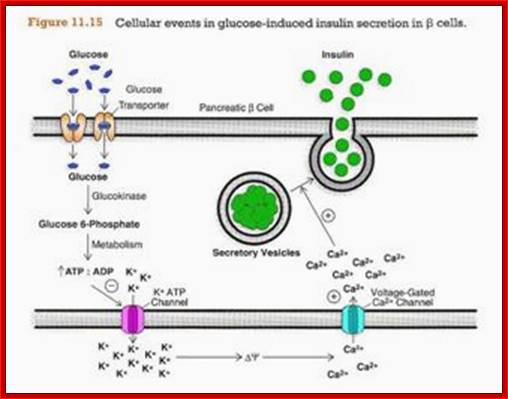
Glucose entry is through glucose transporter 2 (GLUT2). Glut2 is regulated by the concentration blood sugar. If the circulating sugar is more than 4-5mM the GLUT2 channels open, otherwise they remain closed. Entry of glucose is also sensed by Glucokinase (is the key enzyme) that acts as a sensor that leads to opening of K + channels and that leads to opening of Ca+ channels that leads to the release of insulin granules from the cells into circulation. There are five different GLUT transporters.. The above figure shows how the drug Rosiglitazone is responsible for the release of insulin from the cells in diabetic patients.
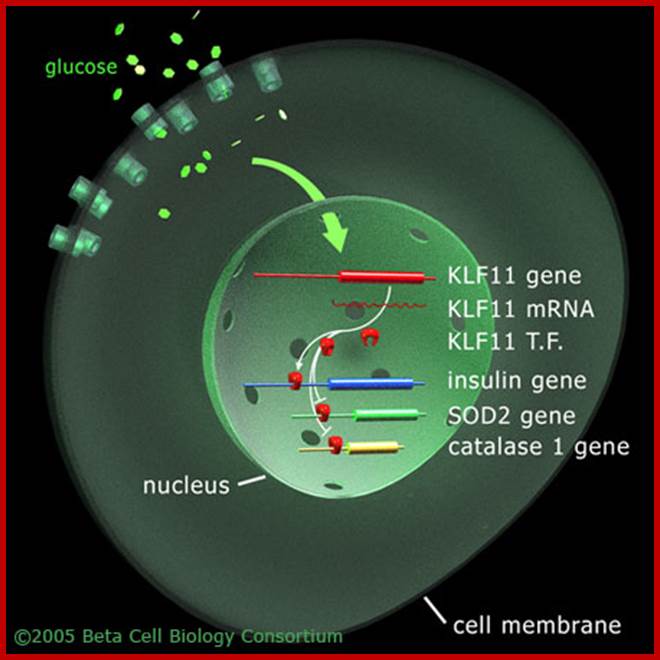
Shown is the pancreatic β-cell. The uptake of glucose initiates the TGF-β signaling pathway to activate transcription factor KLF11. KLF11 then upregulates insulin gene expression and also downregulates the transcription activities of the SOD2 and catalase 1 genes, as studied Neve and colleagies (2005).;KLF regulates Insulin gene expression; Beta cell biology; http://www.betacell.org/
Entry of glucose from cytosol has two effects, one release of insulin into circulation and the second is activation insulin gene itself. This activation is through KLF11 gene product. The insulin gene perse consists of extended promoter region with TATAA box and upstream A4, A3, C1 and E1. Glucose entry has short term effect on insulin synthesis and also it has long term effect where insulin is synthesized and stored.
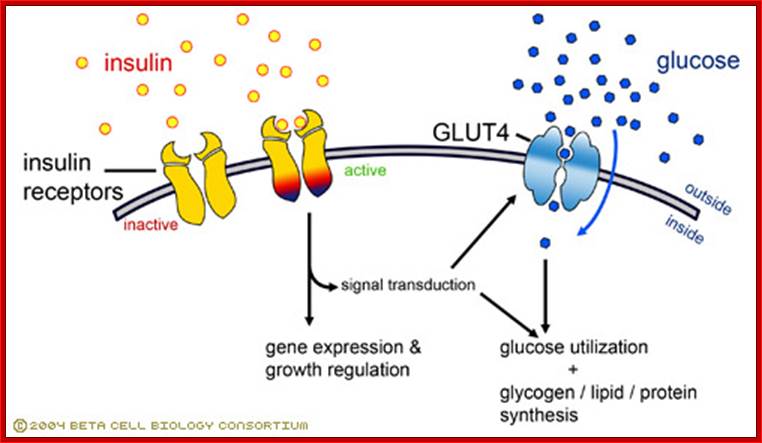
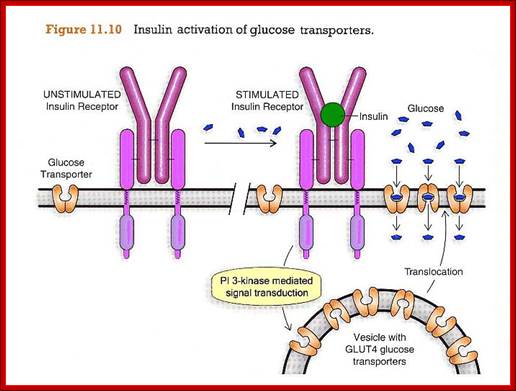
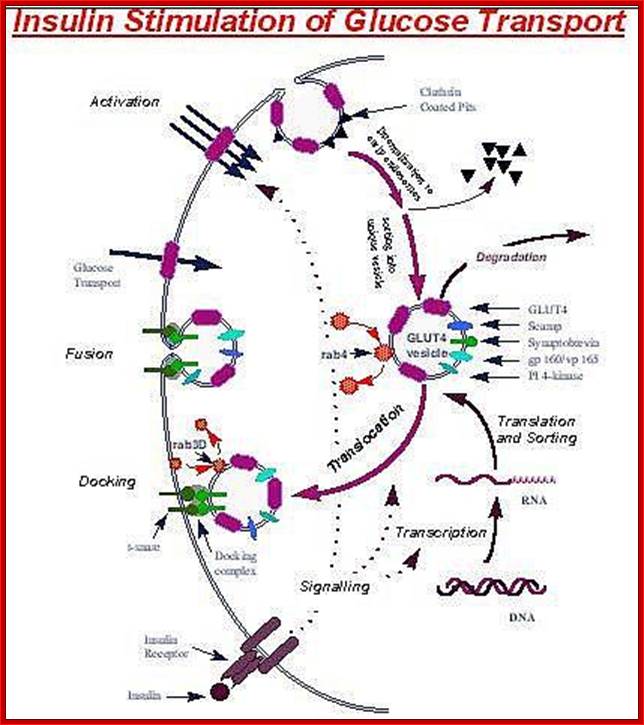
Insulin-mediated glucose uptake
Insulin binding to the insulin receptor induces
a signal transduction cascade which allows the glucose transporter (GLUT4) to
transport glucose into the cell. http://www.betacell.org/
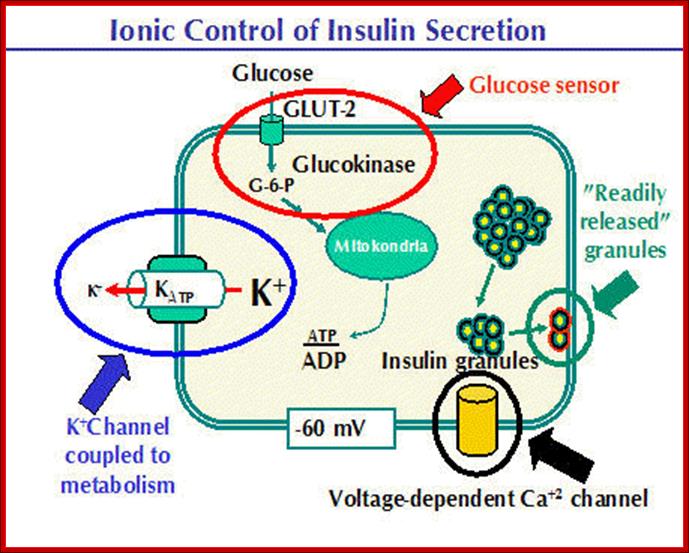
The basic functions and physiology of the beta cell are relatively well understood. A model of the beta cell showing the basic components for insulin secretion is presented below. A glucose "sensor" mechanism, a metabolic coupling to potassium channels to control plasma membrane potential and a voltage dependent Ca++ channel are required to link blood glucose levels to insulin secretion. Insulin containing granules are found in a reserve pool and a "readily released" pool. http://www.medbio.info/
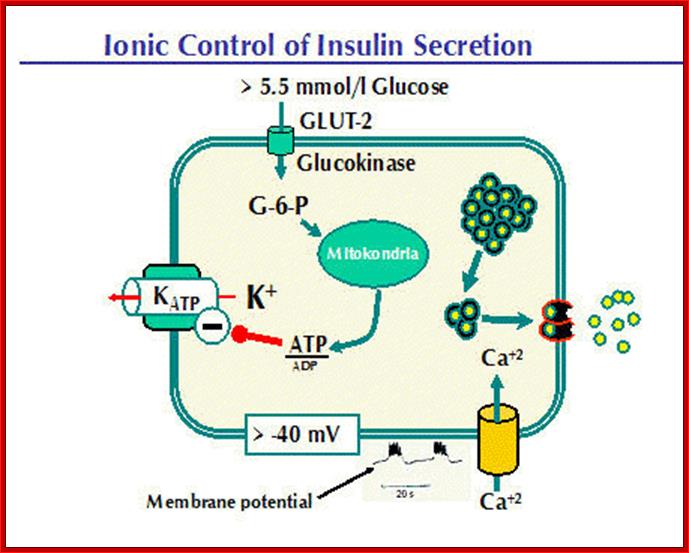
Insulin Secretion is Controlled by Blood Glucose Levels.This process becomes clearer when we examine some examples. Let us look at glucose levels that lead to release of insulin first. GLUT2 and glucokinase are activated when blood glucose increase to about 5.5 mmol/l.Aerobic glycolysis will drive the ATP/ADP ratio upwards. The ATP produced inhibits the KATP channel, thus reducing the flow of potassium ions from the beta cell. As a result, the cell becomes increasingly depolarized. Look at the recording of membrane potential. Slow depolarization waves are initiated as the membrane potential falls. Action potentials occur at the tops of these waves. Insulin secretion is pulsatile, the hormone being secreted in bursts that occur simultaneously with the action potentials. Calcium causes exocytosis from the "rapidly released pool" and migration of insulin-containing granules from the "reserve pool" to the cell membrane where they are "docked" and energized.
http://www.medbio.info/
http://www.medbio.info/
The Voltage-Dependent K+ Channel (Kv channel).
Insulin release from beta cells is graded, controlled by the intracellular K+ concentration. As described above, flux of K+ fromthese cells is believed to be controlled by the KATP channel. However, there are several other potassium- carrying pores in beta cells and the intracellular concentration of K+ reflects the balance between these.
The most interesting today is perhaps the Kv channel. This pore system is voltage-dependent. It opens following depolarization of the cell membrane, in a manner similar to the voltage dependent Ca++ channel (VDCC channel).
While depolarization of the cell membrane follows inhibition of the potassium releasing channel, repolarization is the result of activation of a channel which ships potassium out of the cell! The potassium (K+) level in the beta cell (and, therefore, the membrane potential) is dependent upon the balance between these two port systems. The rate of insulin secretion is, therefore, also dependent upon the balance between these K+ transport mechanisms.
Activity of Kv channels is subjected to control by hormones acting through G-proteins, adenyl cyclase and protein kinase A. Phosphorylation of the Kv channel inhibits K+transport. Kv is also inhibited by NADPH which is formed through glucose metabolism. Glucagon-like protein 1 (GLP-1), which stimulates insulin secretion, seems to act through Kv. The figure above, slightly modified from MacDonald’s review article, summarizes the potassium channel theory
Intracellular Sources of Ca++ Influence Ca++Influx and Insulin Release:
Part of the actions of incretins appear to follow cAMP initiated liberation of intracellular Ca++ which, in turn, triggers a further "Ca++ -induced Ca++ release" or "CICR". The point to note is that insulin release from ß-cells depends on Ca++ initiated action potentials and thatthe amount of insulin released is dependent upon the magnitude and frequency of these potentials. The careful and precise regulation of blood glucose levels found in most people is absolutely dependent upon integration of a large number of factors; glucose sensing mitochondrial metabolism, cyclic AMP levels, and the levels of K+ and Ca++. These points are discussed in detail by Patrick Rorsman, George Holz (1) , George Holz (2) in a series of publications. (Click on their names to come further). The precise mechanisms which lie back of their findings are yet to be well described. However, it appears that genetic variations play a major role in this strict multifactorial coordination. It would appear that a certain degree of disparity is acceptable. However, the metabolic consequences of overweight and aging can throw a genetically stressed situation out of balance and lead to development of glucose intolerance and type 2 diabetes. New approaches to control of diabetes may be expected from these studies.
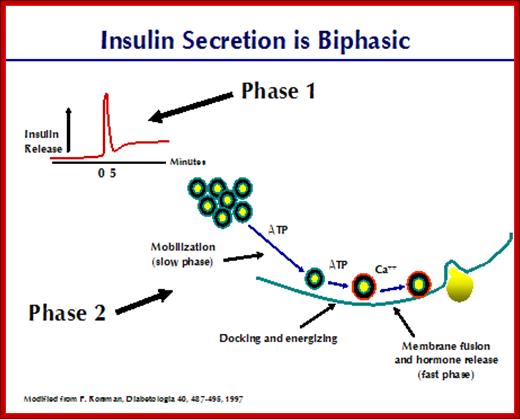
Insulin secretion Biphasic:
http://www.medbio.info
Ingestion of sugar is followed by a biphasic insulin release. A very rapid rise in insulin secretion is seen within minutes after administration of a glucose load. A rapid fall in secretion follows, then pursued by a long-lasting slow release of the hormone. The first top results from exocytosis of granules from the "readily released" pool and comprises 5-10% of the insulin stored in the beta cells. The sustained slow release is comprised of granules from the "reserve pool". Closer examination of this phenomenon has greatly increased knowledge about insulin secretion. Let us examine these steps closely.
1: A fast phase requiring ATP and Ca++ in which granules in the "readily released pool" are already "docked" and "energized". This is the rapid first phase of insulin release and is depicted in the next figure. This phase of insulin secretion accounts for only about 5% on the total released after a meal.
2: A slow second phase requiring ATP. Here, granules are moved from the "reserve pool" to the "readily released" pool of insulin granules. Docking and energizing of the granules are part of this slow second phase. This slow phase is the major contributor to insulin release. Maximal quantities of insulin are released after about 60 minutes.
Pancreatic hormones and metabolic regulation; http://www.trinity.edu/
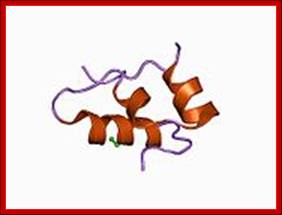
Monomer Insulin; http://en.wikipedia.org/
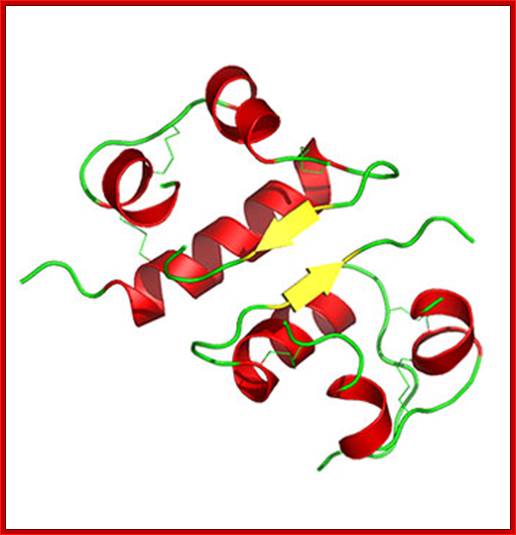
Insulin protein-Dimer; https://www2.chemistry.msu.edu
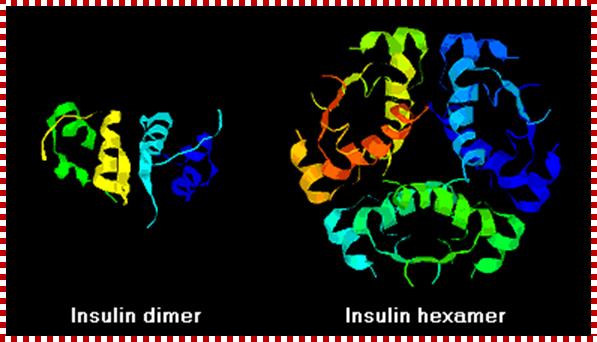
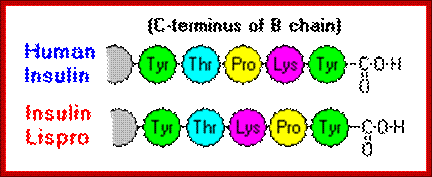
Insulin often , especially recombinant insulin, becomes hexameric complex, which has a problem with the activation of the receptor. The first of these molecules to be marketed - called insulin lispro - is engineered such that lysine and proline residues on the C-terminal end of the B chain are reversed; this modification does not alter receptor binding, but minimizes the tendency to form dimers and hexamers. http://www.vivo.colostate.edu/
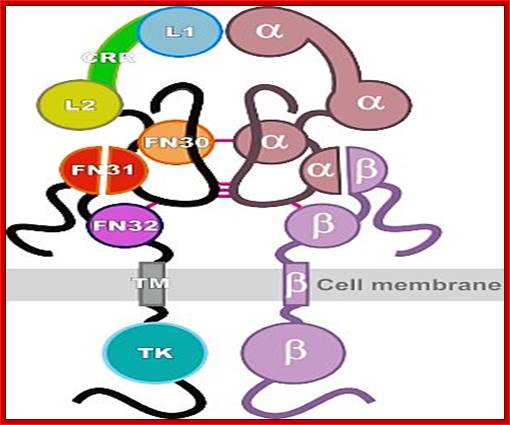
Insulin receptor protein (tetrameric); http://www.phoenixbiotech.net
Insulin receptor protein consists of 2 alpha subunits and two beta subunits. Beta subunits are anchored in the cell membrane, while the alpha subunits dock on to to dimeric beta subunits as covalently linked alpha subunits. Insulin binds to alpha subunit at external surface, that makes dimerization and activation of Tyrosine kinase.

Structural biology of insulin and IGF1 receptors: implications for drug desig; Panel b shows the supra-domain organization of the insulin receptor (modified from Ref. 144). The diagram depicts a 'stretched-out' model of predicted or actual modular structures (for the L1, CR, L2 and TK domains on the basis of X-ray analysis data). The structures of other receptor modules are courtesy of Sir Tom L. Blundell. We do not imply that the actual structure of the receptor has such a stretched-out configuration, which is not supported by recent electron-microscopy studies. The three-dimensional structure of the insulin molecule92 is also shown. The classical binding surface is shown in yellow as van der Waals spheres, and Leu A13 and Leu B17 are shown in red. The insulin backbone is shown in blue. Modelling was carried out using the DS ViewerLite 5.0 (Accelrys). Adapted, with permission, from Ref. 144 © (1999) The Biochemical Society.Modular structure of the insulin receptor; Pierre De Meyts & Jonathan Whittaker; http://www.nature.com/
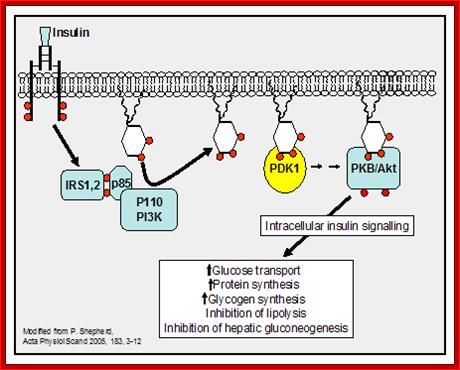
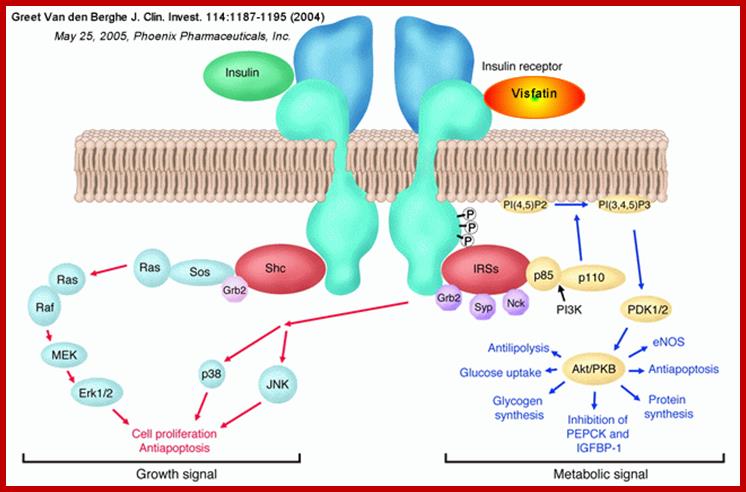
Initial Signaling on Binding of Insulin to the Insulin Receptor; After phosphorylation by the activated insulin receptor protein tyrosine kinase, IRS-1 binds phosphatidylinositol3-kinase (PI3K) that causes phosphorylation of the 3'OH on phosphatidyl inositol (PI) in the inner leaflet of the membrane to form PI(3)P. PI3K is a member of a family of kinases that phosphorylates pI. The metabolic pathway centered on pI3K is one of the most mutated in human cancers. PI(3)P in turn recruits to the membrane other inactive kinases, phosphoinositide-dependent kinase 1, PDK1 and Akt, also known as PKB. ;http://employees.csbsju.edu/
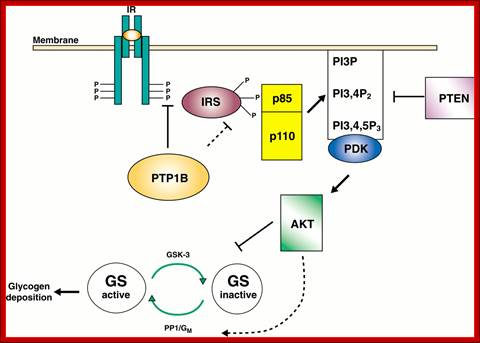
The kinase p-lates IRS proteins. The insulin receptor activates RAS pathway, PI3 kinase pathway leads to the activation of PKB pathway. Activated insulin receptor p-lates IRS which activate PKB and PKC. Insulin stimulated reactions are to lower blood sugar level and promote glycogen synthesis. With PKB kinase acting, intracellular glucose transporters (channel protein) located in cytoplasm as clusters, associated with membranes, are activated and move toward the cell membranes and fuse to provide glucose transport facility.

http://circabook.com
The fusion of Glut4 containing vesicles are marked by the presence of V-SNAREs; even the cell surface membrane also contains tSNAREs. The vescicle with V-SNAREs dock with membranes with tSNAREs thus they fuse with one another and GLUT transporters become active for the transport of sugars in to the cells.
In liver and muscle cells insulin also stimulates glycogen synthase(GS), which uses UDP-glucose to synthesize glycogen. In the absence of insulin GS-kinase3 p-lates GS and makes it inactive, but plates Protein kinase-B (PKB), which in turn activates GSK3 and makes it active, thus GSK inhibition of glycogen synthase is relieved and promote GS activity.
Insulin activated receptor is a tyrosine protein kinase. Activation of the receptor leads to activation of PI3K which activates PDK which activates PKB/Akt that has a ramifying cascading action that can have effects on gene expression.
https://www.pinterest.com
http://healthvenue.net/
Notch signaling Path way:
Notch and Delta proteins are involved in cell differentiation both in vertebrates and invertebrates. Both are pre-transmembrane proteins and contain EGF like exoplasmic domain. They are involved in lateral inhibition where adjacent cells assume different fates. In this progenitor cells (undifferentiated) are prevented from developing into neuronal cells.
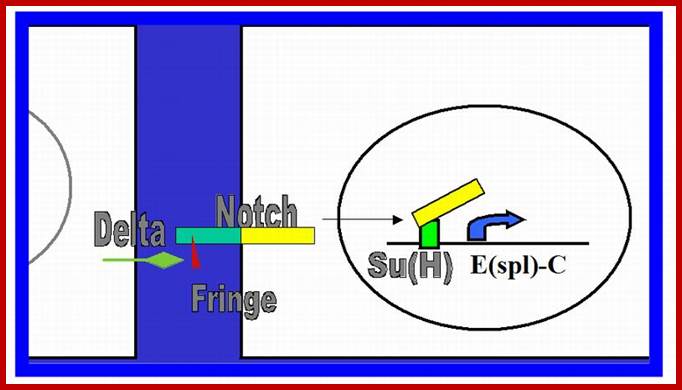
https://wn.com/notch_signaling_pathway
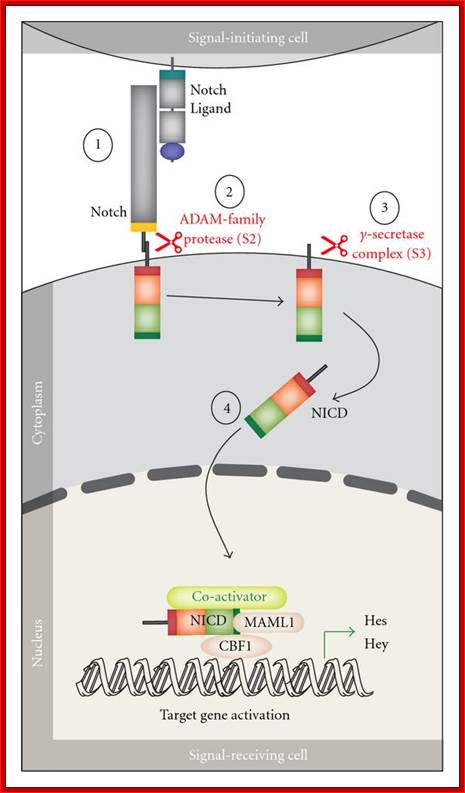
Notch canonical signalling pathway. The figure shows an overview of the canonical Notch signalling pathway (adapted from [8]). The signalling is initiated by ligand binding to Notch receptor (1). This binding initiates two subsequent proteolytic cleavages of the Notch receptor by the ADAM family protease (2) and the γ-secretase, respectively (3). The Notch IntraCellular Domain (NICD) is thereby released to the cytoplasm (4) and translocates to the nucleus where NICD heterodimerizes with the DNA-binding transcription factor CBF1 (also named CSL or Rbp-j). Additional coactivators are also recruited, including mastermind-like proteins (MAML1-3) ultimately leading to induction of transcriptional expression of downstream target genes, including those belonging to the Hes and Hey families. http://www.hindawi.com/
Notch protein synthesized as monomeric membrane protein is transferred to ER, where it binds to Presenilin. It is multipass membrane protein. This complex moves into golgi complex and then they are exported to PM through secretory vesicles. While in golgi the notch protein undergoes cleavage to generate an extracellular and transmembrane subunits. Both are noncovalently with each other. This happens in the absence of delta protein. But in the presence of delta on the other cell binding of notch to delta triggers further cleavage in responding cell. Second cleavage is catalyzed by Presenilin, thus cytosolic side of the notch is released which ultimately translocate into the nucleus. Such signal induced regulated intramembrane proteolysis (RIP) also occurs in response to high cholesterol and to the presence of unfolded protein in ER. This triggers expression of a gene leading to the exit of such proteins into cytoplasm and degradation.
In drosophila the released intracellular segment complexes with DNA binding protein called suppressor hairless (su(H) and stimulates transcription of many gene whose net effect is to influence the determination of cell fate during development. One of the proteins increased in this manner is notch itself. And delta protein production is reduced. Reciprocal regulation of the receptor and ligand is an essential feature of the interaction between initially equivalent cells, that causes cells to assume different cell types. Presenilin (PS1) was identified as autosomal dominant mutant gene for Alzheimer’s’ disease. In this disease pathological change is due to accumulation of amyloid plaques in the brain. The amyloid plaques contain aggregates of Ab42 peptides (42a.a). these are derived form proteolytic cleavage of APP (amyloid precursor protein). It is a cell surface protein in neuron whose function not discerned. APP is cleaved in ways. Early cleavage takes place in the exoplasmic domain, catalyzed by alpha and beta secretase which involves membrane bound metallo proteins. This cleaves notch and generates 26a.a peptides harmless. The b-secretase pathway generates the pathological Abu2. Missense mutation in Presenilin is involved in Alzheimer disease due to plaque formation and neuronal cell death.
Even in C. elegans the role of notch has been detected. Mutation in Presenilin gene cause developmental defects. In mammalian cells signal induced intramembrane proteolysis does not occur. Presenilin is al large complex containing many integral membrane proteins within its membrane spanning sequence region. PS1 contain two aspartate residue resembling water soluble aspartyl residues resembling aspartyl proteases. Mutation in aspartate residues in PS1 abolishes its ability to stimulate cleavage of notch and gamma secretase cleaves APP, indicating some protease is involved.
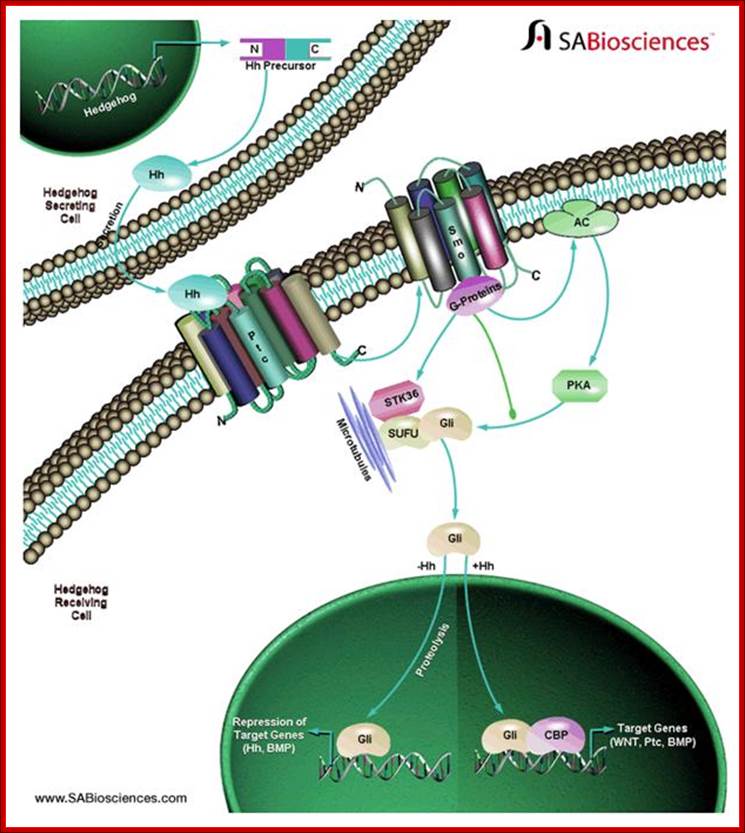
Hedgehog signal pathway:
Hedgehog signals perform a crucial role in developmental process. Hedge hog protein is synthesized as a precursor, which is processed and released. Binding to its receptor it induces signal transduction. The Hh binds to a multipass protein receptor and that leads to the activation of adenyl cyclase; that leads to the activation of PKA. In course of their activity, the signal induced down stream reactions lead to transcriptional activation of specific genes required for development. A very good example is the development of segmental groove in drosophila larval development.
Secreted Hh proteins influence responsive target cells by binding and antagonizing the functional patched (Ptch), a transmembrane receptor that blocks the activity of a signaling effector called Smoothened (Smo). The Hh mediated derepression of Smo leads to reprogramming of gene expression in target cells. Hedgehog aberrant gene lead to skin tumors called basal cell carcinomas. Deregulation of Hh pathway can lead to tumors in brain, lungs, prostrate and gastrointestinal tracts. Hedgehog signaling is under tight spatial and temporal control during embryogenesis.

http://www.lipidmaps.org/
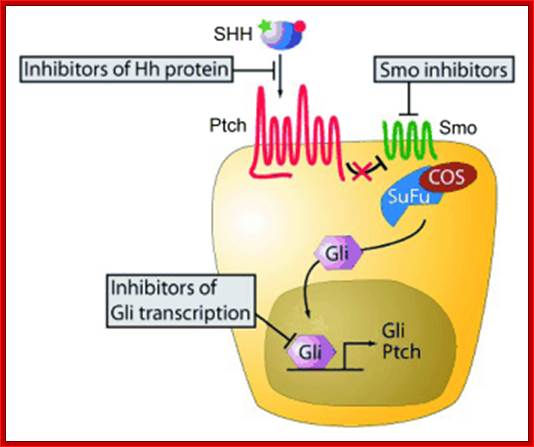
New small-molecule inhibitors of the Hedgehog signaling pathway; Components of the Hh signaling pathway and molecular sites targeted by Hh pathway inhibitors.http://www.otavachemicals.com/
Wnt signaling Pathway:
Wnt proteins are a family of cysteine rich glycoproteins. There 19 Wnt genes in humans. Wnt genes initiates signaling through the binding to Frizzled receptors, which contain several membrane-pass domains. Among the ten identified, three branches have been little understood- beta catenin pathway, the planar cell polarity pathway and Wnt/Ca pathway. Signaling specificity is achieved by specific expression of different frizzled receptors, which can form homo or hetero oligomers.
Beta catenin pathway: In the absence of Wnt , the signaling pool of beta catenin is low because of degradation by ubiquitin-proteasomal pathway. But on Wnt signaling disheveled prevents b-catenin degradation. Frodo and beta-arrestin act synergistically with disheveled. The stabilized b-catenin enters the nucleus and associates with T-cell factor (TCF)/ lymphoid enhancer factor (LEF). The binding leads to the expression of target genes such as cyclinD1, PPARD and twin. In the absence of Wnt DNA histone deacetylation factors prevent the expression of the said genes.
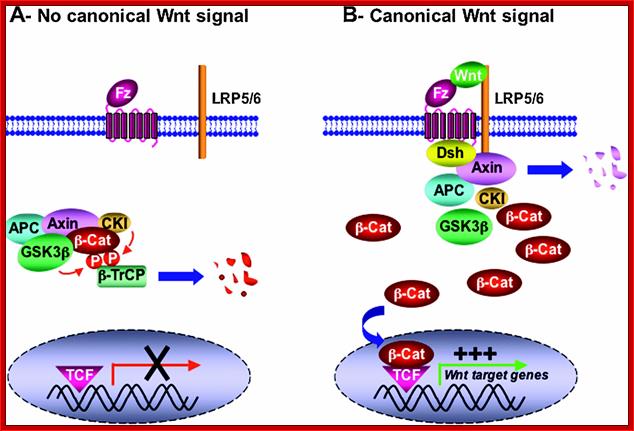
“Figure 4 Simplified overview of the canonical Wnt signalling pathway (adapted from He et al., 2003) (A) In the absence of Wnt ligand, b-catenin is sequestered in a multiprotein degradation complex containing the scaffold protein Axin, the tumour suppressor gene product APC, as well as the kinases CKI and GSK3b, among others. Upon sequential phosphorylation, b-catenin is ubiquitinated by the b-TrCP–E3-ligase complex and subsequently degraded by the proteasome machinery. There is no transcription of Wnt target genes. (B) Wnt ligand associates with Fz and LRP5/6 co-receptors. This in turn can lead to translocation of Axin (and perhaps the whole multiprotein complex) to the plasma membrane through direct interaction with LRP5/6 and Dsh/Fz. Translocation results in Axin degradation and/or dissociation of the multiprotein complex. GSK3b also might be displaced from this complex through Dsh action. b-catenin is then released from the multiprotein complex, accumulates in the cytoplasm in a non-phosphorylated form, and subsequently translocates into the nucleus where by association with TCF/LEF factors it promotes transcription of Wnt target genes.(ref)”http://www.anti-agingfirewalls.com/
Wnt also signals Frizzled receptors to release intracellular Ca+. This pathway includes heterotrimeric G-proteins, PLC and Protein kinase C. In this it is known that NFAT is involved as a TF, which is regulated by calcium/calmodulin proteins
Signal transduction Pathways-A General View:
Signal induced transduction starts with the binding of signals such as light, sound, chemicals, toxins, bacteria etc. The binding of signals to receptors is specific to specific signals. Binding leads to activation which in turn leads down stream activation of several molecules, most of them are proteins or protein kinases. Several of them lead to the nucleus where they activate specific genes specific to signals. Hydrophilic signals initiate signaling at cell surface. But lipophilic signals pass through membranes and bind to cytosolic or nuclear located receptors and induce transcription. The responses can be increase or decrease in metabolic activities, often prevent cell death or cell death. Many of the signal transductions are used for cell proliferation and differentiation. In all these activities kinases play important roles; Kinase can be receptor protein kinases or non receptor kinases, stimulated by RTKs or GPCR. Similarly phosphotases also play counter active roles to kinases, but they are as important as kinases.
Looking at an over view of signal transduction pathways, one finds binding of signal ligands to receptor can lead to single cascade of reactions downstream or lead to multiple pathways or they form a complicated network, which is actually the case.
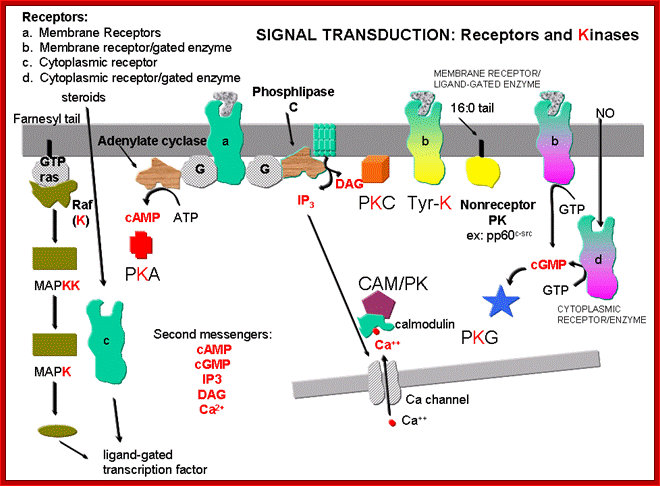
DR. JAKUBOWSKI; http://employees.csbsju.edu/
Activation of multiple signaling pathways by many receptors can lead to the control of many genes independently by different receptors or the same receptor can be opposite effect too. Signaling pathways from the receptor can be routed via Ras/Mapk, or DAG/IP3 and IP3 kinase. They can be involved with Jak and STATs pathways. Or this pathway can be branched from the origin.
Example-, in muscle differentiation, activation of Ras-MAPK inhibits myocyte differentiation into myotubules, where as IP3 kinase pathway promotes differentiation. Initiation of tissue specific signaling pathways by stimulation of the same receptor in different cells is exemplified by EGF receptor activation. EGF stimulated Ras/MAPK pathway in in differentiation of R7 cone cells in drosophila has parallel in the development of vulva in C.elegans.


http://slideplayer.com
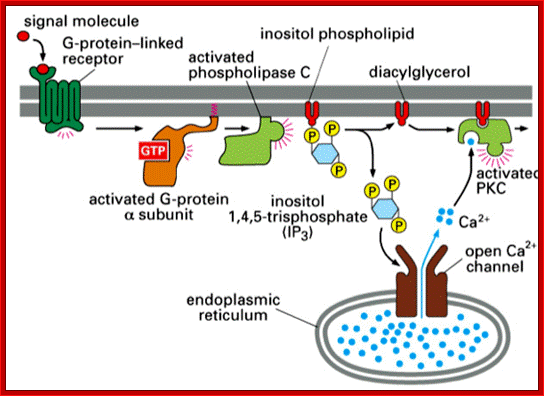
ligand binds GPCR → activate Gq; α subunit activates PLC; PLC breaks down embrane inositol phospholipid → IP3 and diacylglycerol (DAG); IP3 (2nd messenger) activates ER release of Ca2; Ca2+ and DAG bind to PKC which activates it
;http://www.studyblue.com/
In other systems, EGF receptors trigger Ras independent pathway. In C.elegans EGF controls contractility of smooth muscle cells, which is one of the many functions of EGF. This effect regulates extrusion of oocytes from one compartment of the bisexual gonal to the other where they are fertilized.. Coupling of EGF to Ras/MAPK is not required for EGF induced contraction of the gonad.
In C. elegans, EGF–receptor is linked to IP3/DAG pathway. Ligand binding leads to activator of PL-C-gamma, thus increase in IP3, this leads to the release of intracellular Ca+ ions, which leads to muscle contraction.
So in different cell types, stimulation of the same receptor can activate different signaling pathways that produce diverse effects on metabolism and the fate of cell. Environmental stress induces a variety of responses through signal transduced reactions.
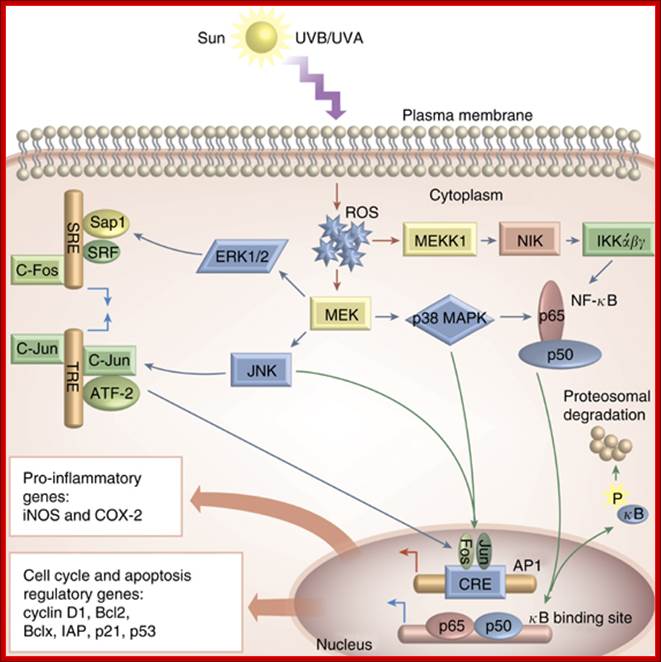
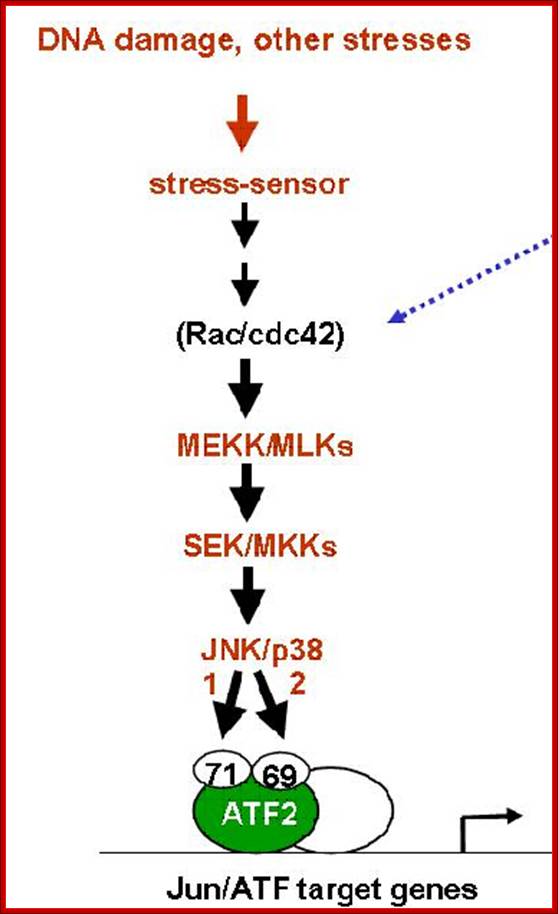
OS-mediated activation of various cell
signaling pathways in the skin. As a result of
UVA/UVB-mediated ROS generation during the pathogenesis of various skin
diseases, a number of signaling pathways are activated. ROS drive activation of
MAPKs, the most important of which are ERK, JNK, and p38 kinases. ERK and JNK
are important in recruiting c-Fos and c-Jun to the nucleus where they activate
the transcription factor AP-1, whereas activation of p38 and inhibitory kappa
kinases (IKK) is important in the transcriptional activation of NF-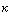 B. Both of these factors are important in regulating the diverse
array of genes, which play key roles in the pathogenesis of inflammation (such
as iNOS, COX-2), and in regulation of cell cycle, proliferation, and apoptosis
(cyclin D1, Bcl2, Bclx, IAP, p21, p53, etc.).Oxidative Stress in the Pathogenesis of Skin Disease; David R Bickers and Mohammad Athar;
http://www.nature.com/
B. Both of these factors are important in regulating the diverse
array of genes, which play key roles in the pathogenesis of inflammation (such
as iNOS, COX-2), and in regulation of cell cycle, proliferation, and apoptosis
(cyclin D1, Bcl2, Bclx, IAP, p21, p53, etc.).Oxidative Stress in the Pathogenesis of Skin Disease; David R Bickers and Mohammad Athar;
http://www.nature.com/
.
Osteoclasts vs Osteoblasts:
Osteoclasts are bone dissolving cells, they are a knind of macrophage cell types that contain dyanamic integrin containing adhesive structures called podosomes in plasma membranes. The alpha Beta3 integrins in podosome is key in initial binding of osteoclasts to the surface of bones. Antibodies to the said integrins block bone resorption. Once osteoclasts bind to bones, they form specialized tight sealing between osteoclasts and bone surface, which creates an extracellular space between them. The osteoclasts secrete corrosive mix of HCL and proteases that dissolve inorganic components of the bone and its proteins. Synthesis and secretion is similar to stomach secretion of HCl. As in stomach gastric juice carbonic unhydrase and an anion antiport proteins are used to generate H+ions within osteoclasts.
On the contrary osteoblasts, a bone forming cell types secrete type-I collagen, which is the major component of bones. Osteoblasts express trimeric cell signaling proteins called RANKL, it is member of TGFalpha family. RANKL is the ligand for RANK cell surface receptor on osteoclast cells. Interaction between them initiate multiple intracellular signaling pathways in osteoclast, this includes NFkB pathway too. These collectively signals induce differentiation of osteoclast and change their shape that permits binding to bones and thus bone resorptiopn takes place. Osteoblasts also produce and secrete soluble decoys receptors called osteoproterigins (OPG) named for its bone protective property. Secreted OPGs bind to RANKL on osteoblasts and prevent RANkL and RANKL receptors interaction, thus inhibit osteoclast activation and bone resorption. Mice with deleted OPG genes have week and porous bones characteristic of massive bone resorption.
Light induced signal transduction:
Light has tremendous effects on cellular activities including light triggered photosynthetic reactions, vision, photoperiodic induction of flowering, circadian rhythm and many others. In all these cells require light absorbing pigments such as chlorophyll/protein complex, phytochrome/protein complex; they in turn execute downstream reactions.

Light induced Calcium2+ release and G activated transduction; http://fig.cox.miami.edu ;http://physrev.physiology.org/


Photoreceptors and circadian clocks are universal mechanisms for sensing and responding to the light environment. In addition to regulating daily activities, photoreceptors and circadian clocks are also involved in the seasonal regulation of processes such as flowering. Circadian rhythms govern many plant processes, including movements of organs such as leaves and petals, stomata opening, stem elongation, sensitivity to light of floral induction, metabolic processes such as respiration and photosynthesis and expression of a large number of different genes." - drawing and quote from Elaine Tobin's Website, UCLA. Phytochromes nd the circadian Clock in plants; http://www.mobot.org/

Mechanism
of flowering by binding between florigen and
its receptors in
shoot apical cells; www.spring8.or.jp

Flowering induction in plants occurs as follows. Florigen synthesized in the leaf is transported through the vascular bundle to the shoot apex, from which flowers come out. In the shoot apical cells, florigen binds to the 14-3-3 receptor in the cytoplasm. The resulting Hd3a-14-3-3 complex is transported into the nucleus and binds to OsFD1 to form a higher complex, the florigen activation complex. This complex binds to the promoter of genes that induce the formation of flower buds, and activates the genes that in turn activate a series of genes that promote flowering. http://www.spring8.or.jp/

Structure and function of florigen and the receptor complex; Ken-ichiro Taoka et al, ;http://www.cell.com
Infection to cell is an invasion of foreign substance which acts like stress. In response to viral or pathogen the receptors such as TLR3 and TLR9 respond and activate several genes down stream for the synthesis of interferons/ cytokines to make cells to defend against pathogens and keep cells in antiviral state till cell-mediated immunity takes over.
Oxidative stress:
UV radiations and even certain biological reactions generate oxygen radicals inside the body which is deleterious to cellular functions. In such situations, cells have inbuilt mechanism to overcome Reactive oxidative stress (ROS). Ros activates a whole lot of cellular kinases, primarily MEK, that leads to activation of p38/MAPK. This leads to p-lation of an inhibitor of NFkB, which gets degraded by ubiquitination followed by proteasome. MAPK also activates Jun/Fos which again activates certain genes whose products can over come oxidative stress.
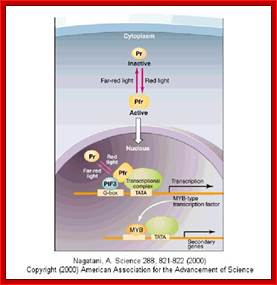
The role of serine kinase activation in oxidative stress−induced insulin resistance. A variety of stimuli, including hyperglycemia, elevated FFA levels, cytokines, and others, increase ROS (and RNS) production and oxidative stress. This results in the activation of multiple stress-sensitive serine/threonine (Ser/Thr) kinase signaling cascades such as IKK-β and others (see text for details). Once activated, these kinases are able to phosphorylate multiple targets, such as the IR and IRS proteins (including IRS-1 and IRS-2). Increased phosphorylation of IR or IRS proteins on discrete serine or threonine sites (pS/T) decreases the extent of insulin-stimulated tyrosine phosphorylation (pY) (52,53). Consequently, the association and/or activities of downstream signaling molecules (e.g., phosphatidylinositol 3-kinase [PI3K]) are decreased, resulting in reduced insulin action (insulin resistance). The protective effects of antioxidants (e.g., LA) on oxidative stress−induced insulin resistance could relate to their ability to preserve the intracellular redox balance (neutralizing ROS) or, analogous to pharmacological agents (e.g., salicylates, p38 MAPK inhibitors), to block the activation of stress-sensitive kinases ;
http;//www.diabetes.diabetesjournals.org
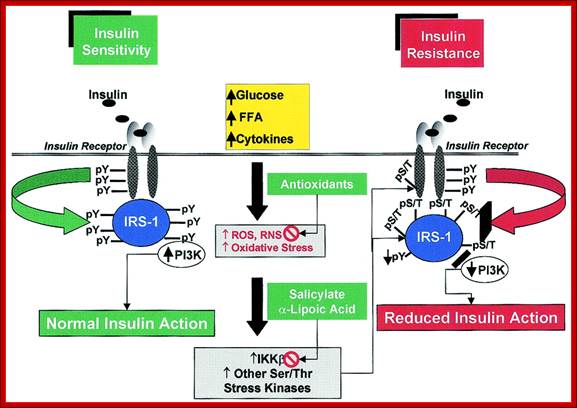
DNA damage induced molecular pathway:
DNA damage has far reaching consequences perhaps leading to cancer, if cells continue divide and redivide. P53 senses the damage through certain kinases (AKT) becomes active. The active p53 induces p21 gene expression. P21 protein is cdk/cyclin inhibitor thus blocks cell proliferation. It also induces apoptosis as a last resort.
https://www.fmhs.auckland.ac.nz
Peg3/Pw1 Is a Mediator between p53 and Bax in DNA Damage-induced Neuronal Death; http://www.jbc.org
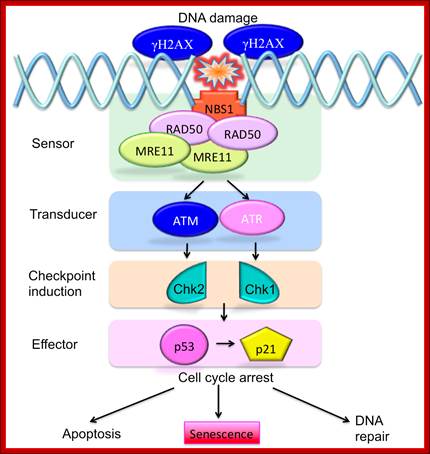
To protect the integrity of their DNA, cells need to be able to sense DNA damage and activate response pathways that coordinate cell cycle progression and DNA repair. Ataxia telangiectasia mutated (ATM) and ATM-Rad3-related (ATR) kinases are important DNA damage checkpoints proteins that transduce signals from the DNA damage sensors to the effector proteins that control cell cycle progression, chromatin restructuring, and DNA repair. ATM and ATR activate other kinases such as Chkl (activated by ATR) and Chk2 (activated by ATM) that phosphorylate and activate the tumour suppressor protein p53. ATM and ATR can additionally enhance p53 activity by directing p53 phosphorylation on Ser15. Activated p53 can halt progression of the cell cycle in the G1 phase, allowing DNA repair to occur and preventing the transmission of damaged DNA to the daughter cells. http://www.intechopen.com/

A contemporary view of the general outline of the DNA damage response signal-transduction pathway; Arrowheads represent activating events and perpendicular ends represent inhibitory events. Cell-cycle arrest is depicted with a stop sign, apoptosis with a tombstone. The DNA helix with an arrow represents damage-induced transcription, while the DNA helix with several oval-shaped subunits represents damage-induced repair. For the purpose of simplicity, the network of interacting pathways are depicted as a linear pathway consisting of signals, sensors, transducers and effectors. The DNA damage response: putting checkpoints in perspective; Bin-Bing S. Zhou & Stephen J. Elledge; http://www.nature.com
DNA damage stress sensor induced pathway
Enhanced DNA binding capacity on up-regulated epidermal wild-type p53 in vitiligo by H2O2-mediated oxidation: a possible repair mechanism for DNA damage: Mohamed M. A. E. L. SalemMohamed M. A. E. L. Salem et al.
Scheme shows up-regulated wild-type p53 as proposed main conductor of ROS-mediated DNA damage/repair in vitiligo. ROS (H2O2/ONOO−)-mediated DNA damage in vitiligo leads to increased 8-oxoG levels in the epidermis and in plasma (Fig. 2A⇓ ). Phosphorylation is a crucial step for p53 stabilization to perform its function as a transcription factor (61⇓ , 103⇓ , 104)⇓ . In this context, it has been shown that phosphorylation of p53 protein is initiated by DNA damage. Both ATM and PCAF are induced by DNA damage (104⇓ , 105)⇓. Increased wild-type p53 levels in vitiligo are phosphorylated on ser9 and ser15, proving, in turn, functionality of overexpressed ATM in vitiligo. Moreover, increased epidermal PCAF is functioning, as shown by the presence of acetylated lysine residues at positions 373 and 382 (Fig. 3⇓ ). Therefore, these steps further support functioning stabilized activated wild-type p53 in vitiligo (22)⇓ . Under normal conditions, p53 is degraded by p90MDM2 (106)⇓. The isomer p76MDM2 antagonizes the action of p90MDM2, preventing binding of p90MDM2 to p53, stopping, in turn, degradation of p53 (28)⇓ . Our results identified for the first time high epidermal p76MDM2 levels in vitiligo (Fig. 6B⇓ ), while p90MDM2 expression is not affected in this compartment (Fig. 6A⇓ ). Functioning stabilized activated p53 leads to transcription of Gadd45α, p21, and PCNA (Fig. 4A, B⇓ ). Both p21 and Gadd45α induce cell cycle arrest, and both signals are up-regulated in vitiligo. Oxidative DNA damage is repaired via BER. This step is initiated by glycosylase hOgg1, which can excise 8-oxoG (50⇓ , 51⇓ , 107)⇓ . Expression levels of hOgg1 are not affected in vitiligo despite the presence of oxidative stress via H2O2 and ONOO− (Fig. 2B⇓ ). Depending on the type of DNA damage, the repair pathways can involve either short- or long-patch BER, although these pathways can switch between each other (108⇓ 109⇓ 110)⇓ . PCNA binds to DNA polymeraseδ and works as a processivity factor for this enzyme, which together with FEN1 is involved in long-patch BER (110⇓ 111⇓ 112⇓ 113)⇓ . Notably, we were unable to detect epidermal DNA polymeraseδ and FEN1 in our patients (data not shown), but we found up-regulated epidermal APE1 and DNA polymeraseβ levels (Fig. 2B⇓ ). This result favors DNA repair viashort-patch BER in vitiligo. One indicator for epidermal DNA damage after sun exposure is the presence of sunburn cells (19)⇓ . Low numbers of sunburn cells despite several sunburns could support increased DNA repair in these individuals (19)⇓ . Notably, PCNA can interact directly with APE1 and DNA polymerase β (114)⇓ . Moreover, hOgg1, APE1, and DNA polymeraseβ are directly regulated by p53 (115)⇓ . Therefore, it is tempting to conclude that oxidative DNA damage in vitiligo can be repaired by short-patch BER and possibly in cooperation with a specified long-patch BER in the absence of FEN1 and DNA polymeraseδ. Epidermal programmed cell death in vitiligo is unlikely, because up-regulated Bcl-2 (Fig. 5⇓ ) favors antiapoptotic activity that is further supported by decreased cytochrome c and caspase 3 levels (Fig. 5⇓ ). These new findings are further supported by unremarkable levels of the proapoptotic stimulus BAX and low epidermal acetylcholine esterase levels/activities in vitiligo, as shown earlier (9⇓ , 23)⇓ . Notably, DNA binding capacity of p53 is enhanced by H2O2, and it can abolish or prevent ONOO−-induced abrogation of DNA binding on p53, as shown within this study (Fig. 8A, B⇓ ). Taken together, we propose that patients with vitiligo can efficiently combat epidermal H2O2/ONOO−-induced DNA damage viaincreased activated/stabilized wild-type functioning p53 (Fig. 1A, B⇓ ). These findings, together with enhanced DNA binding capacity, support p53 as a master conductor in the scenario, providing an explanation for the normal incidence of solar-induced skin cancer and low photodamage and premature skin aging in this patient group (19⇓ , 20)⇓ . Mohamed M. A. E. L. Salem et al
Notch signaling Path way:
Notch and Delta proteins are involved in cell differentiation both in vertebrates and invertebrates. Both are pre-transmembrane proteins and contain EGF like exoplasmic domain. They are involved in lateral inhibition where adjacent cells assume different fates. In this progenitor cells (undifferentiated) are prevented from developing into neuronal cells.
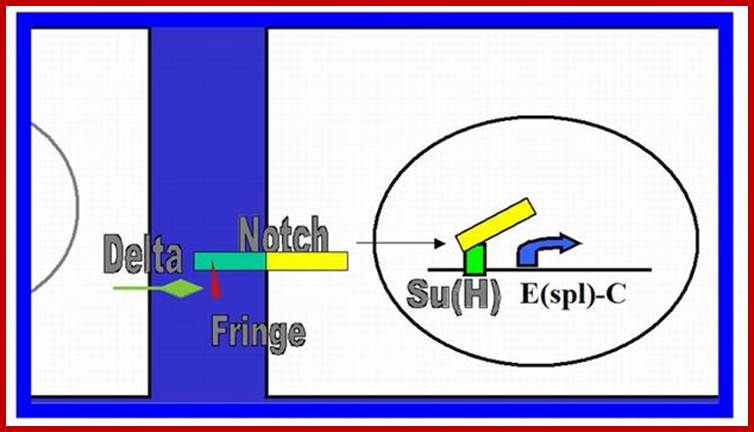
Notch protein synthesized as monomeric membrane protein is transferred to ER, where it binds to Presenilin. It is multipass membrane protein. This complex moves into Golgi complex and then they are exported to PM through secretory vesicles. While in Golgi the notch protein undergoes cleavage to generate extracellular and transmembrane subunits. Both are noncovalently with each other. This happens in the absence of delta protein. But in the presence of delta on the other cell binding of notch to delta triggers further cleavage in responding cell. Second clveage is catalyzed by Presenilin, thus cytosolic side of the notch is released which ultimately translocate into the nucleus. Such signal induced regulated intramembrane proteolysis (RIP) also occurs in response to high cholesterol and to the presence of unfolded protein in ER. This triggers expression of a gene leading to the exit of such proteins into cytoplasm and degradation.
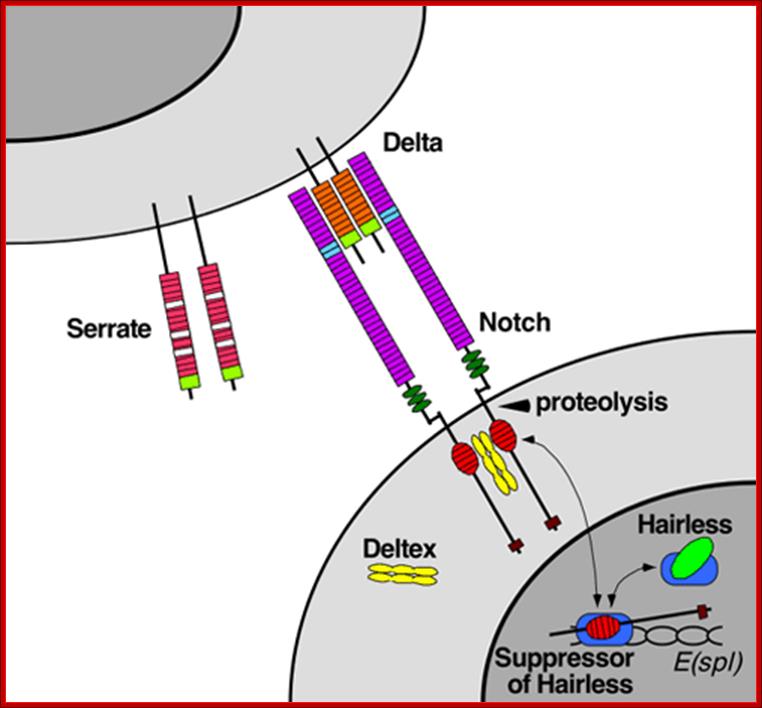
In drosophila the released intracellular segment complexes with DNA binding protein called suppressor hairless (su(H) and stimulates transcription of many gene whose net effect is to influence the determination of cell fate during development. One of the proteins increased in this manner is notch itself. And delta protein production is reduced. Reciprocal regulation of the receptor and ligand is an essential feature of the interaction between initially equivalent cells that causes cells to assume different cell types. Presenilin (PS1) was identified as autosomal dominant mutant gene for Alzheimer’s’ disease. In this disease pathological change is due to accumulation of amyloid plaques in the brain. The amyloid plaques contain aggregates of Ab42 peptides (42a.a). These are derived from proteolytic cleavage of APP (amyloid precursor protein). It is a cell surface protein in neuron whose function not discerned. APP is cleaved in ways. Early cleavage takes place in the exoplasmic domain, catalyzed by alpha and beta secretase which involves membrane bound metallo protein. This cleaves notch and generates 26a.a peptides harmless. The b-secretase pathway generates the pathological Abu2. Missense mutation in Presenilin is involved in Alzheimer disease due to plaque formation and neuronal cell death.
Even in C.elegans the role of notch has been detected. Mutation in Presenilin gene cause developmental defect. in mammalian cells signal induced intramembrane proteolysis does not occur. Presenilin is al large complex containing many integral membrane proteins within its membrane spanning sequence region. PS1 contain two aspartate residues resembling water soluble aspartyl residues resembling aspartyl proteases. Mutation in aspartate residues in PS1 abolishes its ability to stimulate cleavage of notch and gamma secretase cleaves APP, indicating some protease is involved.
Hedgehog signal pathway:
Hedgehog signals perform a crucial role in developmental process. Hedge hog protein is synthesized as a precursor, which is processed and released. Binding to its receptor it induces signal transduction. The Hh binds to a multipass protein receptor and that leads to the activation of adenyl cyclase; that leads to the activation of PKA. In course of their activity, the signal induced down stream reactions lead to transcriptional activation of specific genes required for development. A very good example is the development of segmental groove in drosophila larval development.
Secreted Hh proteins influence responsive target cells by binding and antagonizing the functional patched (Ptch), a transmembrane receptor that blocks the activity of a signaling effector called Smoothened (Smo). The Hh mediated derepression of Smo leads to reprogramming of gene expression in target cells. Hedgehog aberrant gene lead to skin tumors called basal cell carcinomas. Deregulation of Hh pathway can lead to tumors in brain, lungs, prostrate and gastrointestinal tracts. Hedgehog signaling is under tight spatial and temporal control during embryogenesis.

Hedge Hog
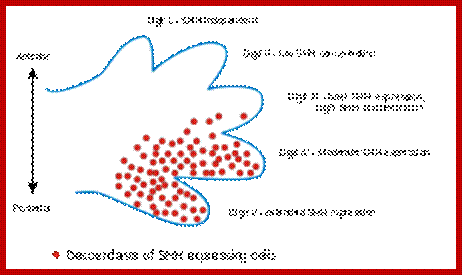
http://wikivisually.com
Its fingers
Wnt signaling Pathway:
Wnt proteins are a family of cysteine rich glycoproteins. There 19 Wnt genes in humans. Wnt genes initiates signaling through the binding to Frizzled receptors, which contain several membranes, pass domains. Among the ten identified, three branches have been little understood- beta catenin pathway, the planar cell polarity pathway and Wnt/Ca pathway. Signaling specificity is achieved by specific expression of different frizzled receptors, which can form homo or hetero oligomers.
Beta catenin pathway: In the absence of Wnt , the signaling pool of beta catenin is low because of degradation by ubiquitin-proteasomal pathway. But on Wnt signaling disheveled prevents b-catenin degradation. Frodo and beta-arrestin act synergistically with dishvelled. The stabilized b-catenin enters the nucleus and associates with T-cell factor (TCF)/ lymphoid enhancer factor (LEF). The binding leads to the expression of target genes such as cyclinD1, PPARD and twin. In the absence of Wnt DNA histone deacetylation factors prevent the expression of the said genes. Wnt also signals Frizzled receptors to release intracellular Ca+. This pathway includes heterotrimeric G proteins, PLC and Protein kinase C. In this it is known that NFAT is involved as a TF, which is regulated by calcium/calmodulin proteins
Signal transduction Pathways-A General View:
Signal induced transduction starts with the binding of signals such as light, sound, chemicals, toxins, bacteria etc. The binding of signals to receptors is specific to specific signals. Binding leads to activation which in turn leads down stream activation of several molecules, most of them are proteins or protein kinases. Several of them lead to the nucleus where they activate specific genes specific to signals. The signal such as hydrophilic initiate signaling at cell surface. But lipophilic signals pass through membranes and bind to cytosolic or nuclear located receptors and induce transcription. The responses can be increase or decrease in metabolic activities often prevent cell death or cell death. Many of the signal transduction are used for cell proliferation and differentiation. In all these activities kinases play important roles; Kinase can be receptor protein kinases or non receptor kinases, stimulated by RTKs or GPCR. Similarly phosphotases also play counter active roles to kinases, but they are as important as kinases.
Looking at an over view of signal transduction pathways, one finds binding of signal ligands to receptor can lead to single cascade of reactions downstream or or lead to multiple pathways or they form a complicated network, which is actually the case.
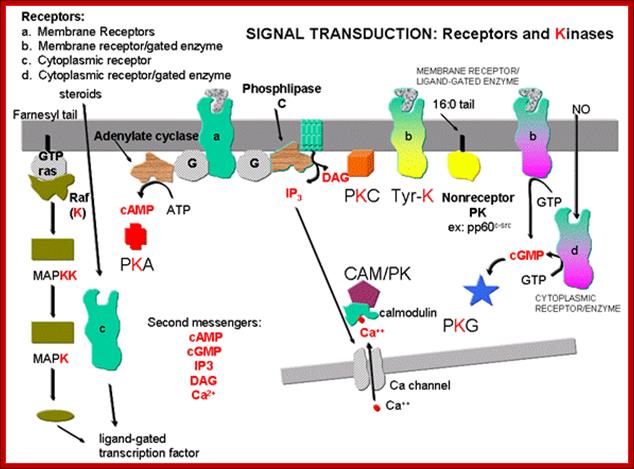
https://www.slideshare.net
Activation of multiple signaling pathways by many receptors can lead to the control of many genes independently by different receptors or the same receptor can be opposite effect too. Signaling pathways from the receptor can be routed via Ras/Mapk, or DAG/IP3 and IP3 kinase. They can be involved with Jak and STATs pathways. Or this pathway can be branched from the origin.
Example-, in muscle differentiation, activation of Ras-MAPK inhibits myocyte differentiation into myotubules, where as IP3 kinase pathway promotes differentiation. Initiation of tissue specific signaling pathways by stimulation of the same receptor in different cells is exemplified by EGF receptor activation. EGF stimulated Ras/MAPK pathway in in differentiation of R7 cone cells in drosophila has parallel in the development of vulva in C.elegans.
In other systems, EGF receptors trigger Ras independent pathway. In C.elegans EGF controls contractility of smooth muscle cells, which is one of the many functions of EGF. This effect regulates extrusion of oocytes from one compartment of the bisexual gonal to the other where they are fertilized.. Coupling of EGF to Ras/MAPK is not required for EGF induced contraction of the gonad.
In C. elegans , EGF–receptor is linked to IP3/DAG pathway. Ligand binding leads to activation of PL-Cgamma, thus increase in IP3, this leads to the release of intracellular Ca+ions , which leads to muscle contraction.
So in different cell types, stimulation of the same receptor can activate different signaling pathways that produce diverse effects on metabolism and the fate of cell. Environmental stress induces a variety of responses through signal transduced reactions.
Osteoclasts vs Osteoblasts:
Osteoclasts are bone dissolving cells, they are a kind of macrophage cell types that contain dynamic integrin containing adhesive structures called podosomes in plasma membranes. The alpha Beta3 integrins in podosome is key in initial binding of osteoclasts to the surface of bones. Antibodies to the said integrins back bone resorptiopn. Once osteoclasts bind to bones, they form specialized tight sealing between osteoclasts and bone surface, which creates an extracellular space between them. The osteoclasts secrete corrosive mix of HCL and proteases that dissolve inorganic components of the bone and its proteins. Synthesis and secretion is similar to stomach secretion of HCl. As in stomach gastric juice carbonic anhydrase and an anion antiport proteins are used to generate H+ ions within osteoclasts.
On the contrary osteoblasts , a bone forming cell types secrete type-I collagen, which is the major component of bones. Osteoblasts express trimeric cell signaling proteins called RANKL, it is member of TGFalpha family. RANKL is the ligand for RANK cell surface receptor on osteoclast cells. Interaction between them initiate multiple intracellular signaling pathways in osteoclast, this includes NFkB pathway too. These collectively signals induce differentiation of osteoclast and change their shape that permits binding to bones and thus bone resorptiopn takes place. Osteoblasts also produce and secrete soluble decoys receptors called osteoproterigins (OPG) named for its bone protective property. Secreted OPGs bind to RANKL on osteoblasts and prevent RANkL and RANKL receptors interaction. Thus inhibit osteoclast activation and bone resorption. Mice with deleted OPG genes have week and porous bones characteristic of massive bone resorption.
Infection to cell is an invasion of foreign substance which acts like stress. In response to viral or pathogen the receptors such as TLR3 and TLR9 respond and activate several genes down stream for the synthesis of interferons/ cytokines to make cells to defend against pathogens and keep cells in antiviral state till cell-mediated immunity takes over.
DNA damage induced molecular pathway:
DNA damage has far reaching consequences perhaps leading to cancer, if cells continue divide and redivide. P53 senses the damage through certain kinases (AKT) becomes active. The active p53 induces p21 gene expression. P21 protein is cdk/cyclin inhibitor thus blocks cell proliferation. It also induces apoptosis as a last resort.

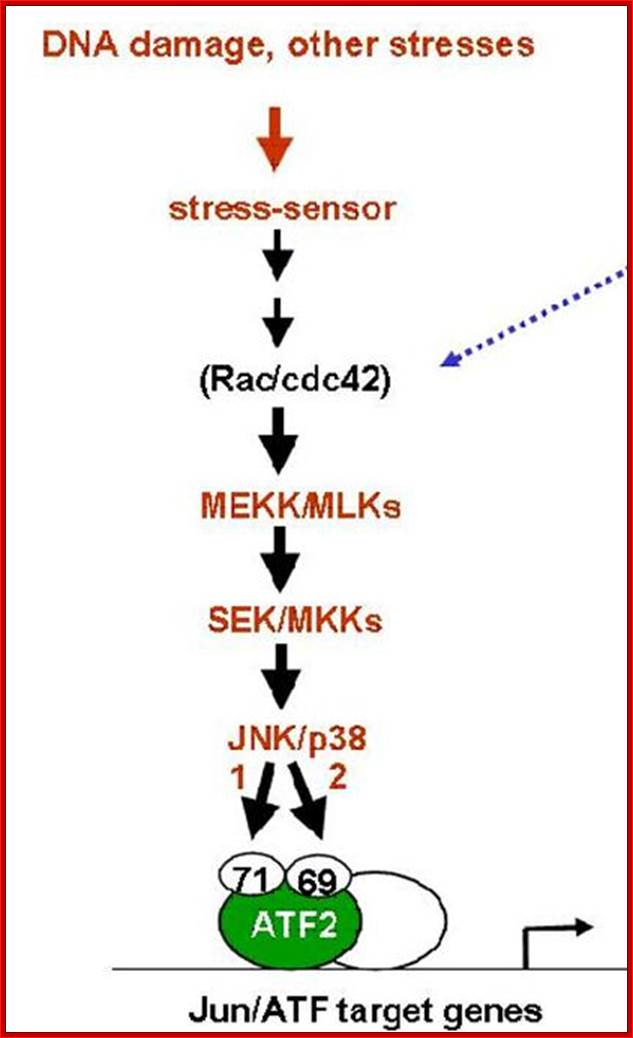
The relationship between Peg3/Pw1 and cell death mediators induced in cortical neurons by DNA damage. The present results demonstrate that Peg3/Pw1 is induced in a p53-dependent manner in response to DNA damage. Previous work from our laboratory demonstrated that p53-mediated cell death was dependent on the presence of the pro-apoptotic Bcl-2 family member Bax (2). An important component of Bax-dependent cell death is its translocation from the cytosol to the mitochondria; http://www.jbc.org/
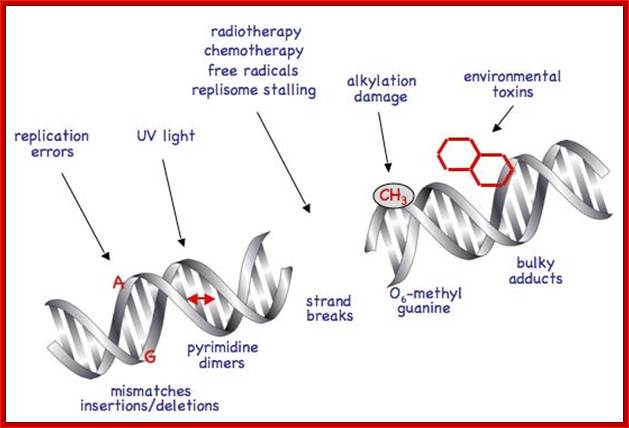
DNA damage stress sensor induced pathway ;https://www.researchgate.net
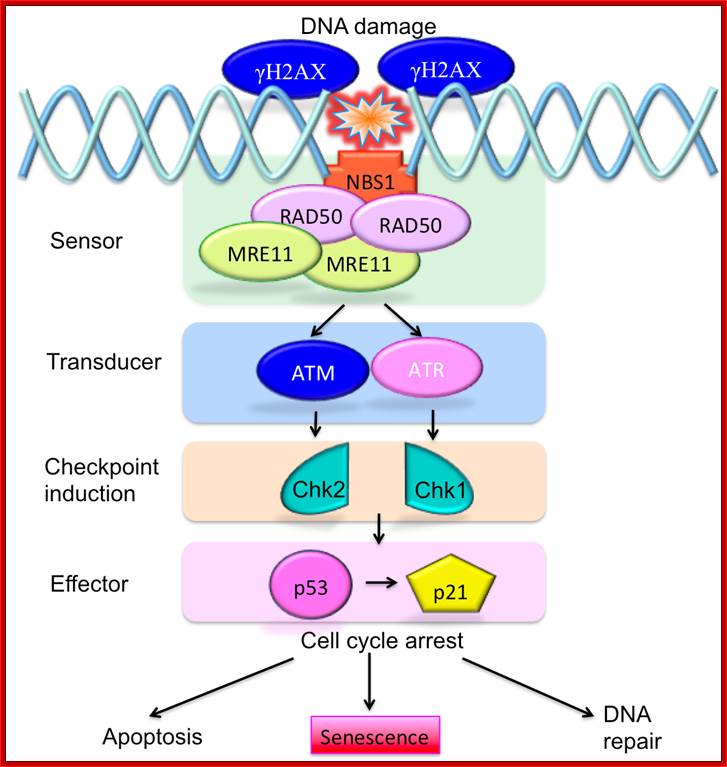
To protect the integrity of their DNA, cells need to be able to sense DNA damage and activate response pathways that coordinate cell cycle progression and DNA repair. Ataxia telangiectasia mutated (ATM) and ATM-Rad3-related (ATR) kinases are important DNA damage checkpoints proteins that transduce signals from the DNA damage sensors to the effector proteins that control cell cycle progression, chromatin restructuring, and DNA repair. ATM and ATR activate other kinases such as Chkl (activated by ATR) and Chk2 (activated by ATM) that phosphorylate and activate the tumour suppressor protein p53. ATM and ATR can additionally enhance p53 activity by directing p53 phosphorylation on Ser15. Activated p53 can halt progression of the cell cycle in the G1 phase, allowing DNA repair to occur and preventing the transmission of damaged DNA to the daughter cells. http://www.intechopen.com/

ATM – Ataxia Telangiectasia protein kinase ( actually it is Pi3k like) once this is activated it tries to recover the damage, if not go to tolerance or apoptosis