Signal transduction 2
Cell_Surface_And_Cytosolic_Receptors
Cell Surface and Cytosol Receptor:
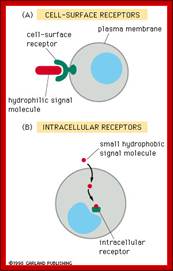
Receptors receive signals that give commands to the cell. There are two major classes of receptors. Receptors are proteins which can be cell surface receptors - embedded in the cell membrane or intracellular receptors - inside the cell.
Class 1: Cell Surface Receptors
Can be divided into several categories:
- Ligand gated Ion channels
- G-protein coupled receptors
- Receptor tyrosine kinases
- Integrins
- Toll-like receptors
Class 2: Intracellular receptors
Can be divided into 2 general categories:
- Cytoplasmic- in the cytoplasm of the cell
- Nuclear- in the nucleus of the cell
A large number of cell surface protein receptors act as transducers, enzyme themselves or associated with enzymes and receptor channels. These receptors consist of exoplasmic ligand binding domain, a single pass transmembrane (helix) domain and an elaborate cytosolic domain which extends deep into cytosol. They don’t interact with trimeric G-proteins. These are six (mainly) classes of receptors in animal systems; Receptor tyrosine kinases (RTK), Receptor tyrosine phosphotases, Receptor serine/threonine kinases, Tyrosine kinases linked- receptors, Receptor guanylate cyclases, and surface proteases. Among them RTKs and Receptor serine threonine kinases (RSK) are more abundant, similar type of receptors are also found in plants called Plant receptor kinases (PRKs). Whether the receptor is GPCR like or RTK like, all of them as they are anchored in membranes, they are always in motion laterally, because the membrane components are fluid like and in constant movement. Often receptors are endocytosed and kept inside the cytosol and when required they are moved back to the cell surface, e.g. Insulin receptor. In many cases where the signal transduction to be downgraded membrane receptors are endocytosed and degraded.

A Cell-surface Receptor. These receptors span the cell membrane and detect chemical signals on the outside of the cell and transmit this detection inside the cell .http://www.bio.miami.edu/; http://www.scq.ubc.ca
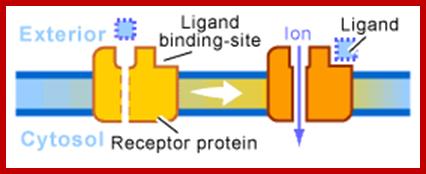
Ion channel receptors; http://www.scq.ubc.ca/

G-protein-Linked Receptor; often involed in desease ex.Cholerahttp://www.scq.ubc.ca

Enzyme linked receptors; http://www.scq.ubc.ca
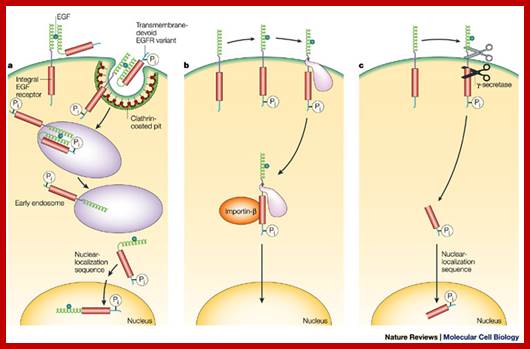
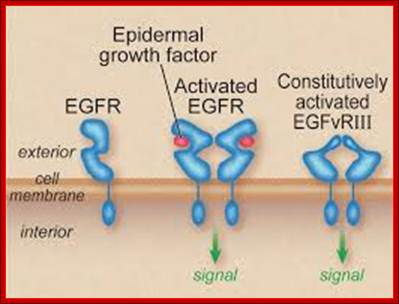
The published data indicate three possibilities. a | A variant of EGFR that lacks the transmembrane segment is internalized with the wild-type receptor5. b | EGFR escapes directly from the membrane and moves to the nucleus using chaperone-like or other accessory proteins6, 7. c | The cytoplasmic domain of EGFR is released by proteolytic cleavage events9, 28. In b and c, only the EGFR monomer is shown. It must be emphasized that none of these three options has yet been shown to occur in intact organisms, although there is proof-of-principle evidence from experimental cellular systems. In addition, these mechanisms might not be mutually exclusive. http://www.nature.com
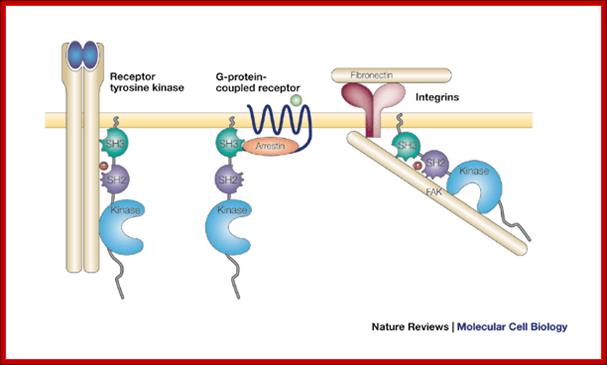
An
example; Src can be activated by several types of cell surface or cytoplasmic
receptor. Shown here are, from left to right, a receptor tyrosine kinase such
as the platelet-derived growth factor receptor; a G-protein-coupled receptor
such as the ![]() -adrenergic receptor; and an integrin bound to extracellular matrix.
In each case, Src is activated by binding of a ligand to the SH2 and/or SH3
domains. In the case of a receptor tyrosine kinase, the SH2 ligand is a
phosphotyrosine residue in the autophosphorylated receptor; in the case of the
-adrenergic receptor; and an integrin bound to extracellular matrix.
In each case, Src is activated by binding of a ligand to the SH2 and/or SH3
domains. In the case of a receptor tyrosine kinase, the SH2 ligand is a
phosphotyrosine residue in the autophosphorylated receptor; in the case of the ![]() -adrenergic receptor, the SH3 ligand is a proline motif in
-adrenergic receptor, the SH3 ligand is a proline motif in ![]() -arrestin bound to the receptor; in the case of an integrin, the SH2
ligand is a phosphorylated tyrosine residue in autophosphorylated focal
adhesion kinase (FAK). Regulation of c-Src by surface receptors. Steven
martin; http://www.nature.com/
-arrestin bound to the receptor; in the case of an integrin, the SH2
ligand is a phosphorylated tyrosine residue in autophosphorylated focal
adhesion kinase (FAK). Regulation of c-Src by surface receptors. Steven
martin; http://www.nature.com/
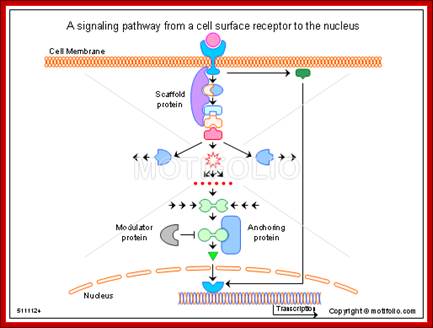
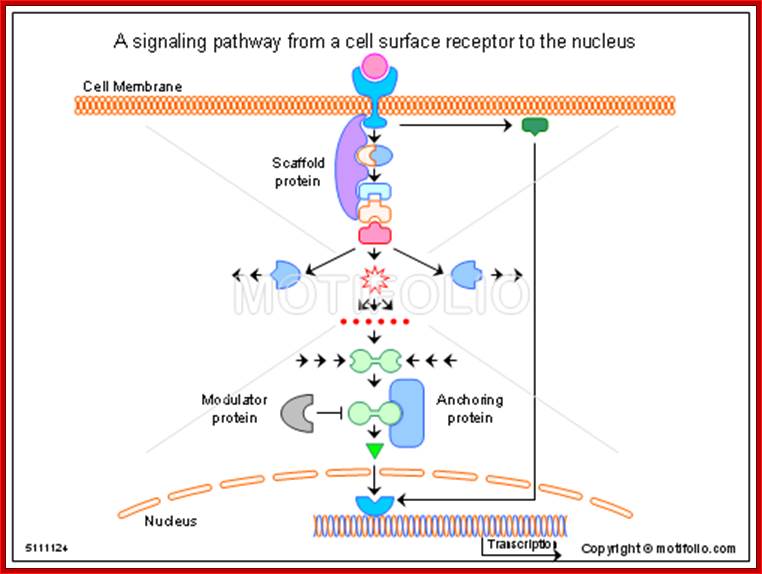
A signaling pathway from a cell surface receptor to the nucleus; Alan Wells & Ulrich Marti; http://www.motifolio.com/
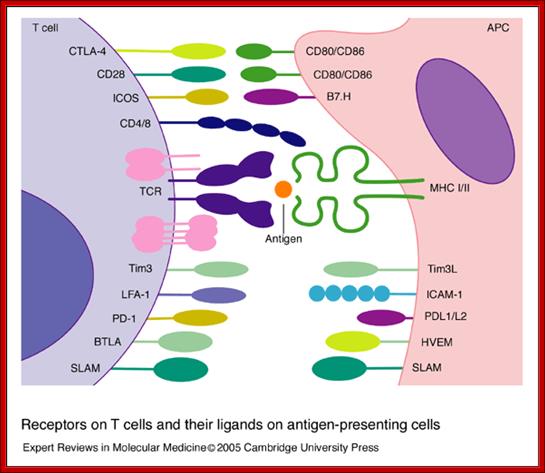
Receptors on T cells and their ligands on antigen-presenting cells. T cells express an array of surface receptors that interact with ligands on antigen-presenting cells (APCs). The T-cell receptor (TCR) and associated CD3 subunits (shown in pink) are engaged by a specific peptide antigen presented by the major histocompatibility complex (MHC). CD4 and CD8 bind to non-polymorphic determinants in class II and I antigens, respectively. Co-signals are provided by CD80/CD86 binding to CD28 and CTLA-4, ICOS binding to B7.H, Tim3 binding to Tim3L, SLAM–SLAM binding, PD-1 binding to PDL1/L2, and BTLA binding to HVEM; other interactions such as LFA-1 binding to ICAM-1 and CD2 binding to CD48/58 (not shown) are important for the adhesion between T cells and APCs. Abbreviations: BTLA, B- and T-lymphocyte attenuator; CTLA-4, cytotoxic T-lymphocyte antigen 4; HVEM, herpesvirus entry mediator; ICAM-1, intercellular cell adhesion molecule 1; ICOS, inducible co-stimulator; LFA-1, leukocyte function-associated antigen 1; PD-1, program death 1 receptor; SLAM, signalling lymphocyte activation molecule; Tim3, T-cell immunoglobulin- and mucin-domain-containing molecule 3. Receptors on T cells and their ligands on antigen-presenting cells; Beverley Wilkinson, Jocelyn S. Downey and Christopher E. Rudd; http://journals.cambridge.org/

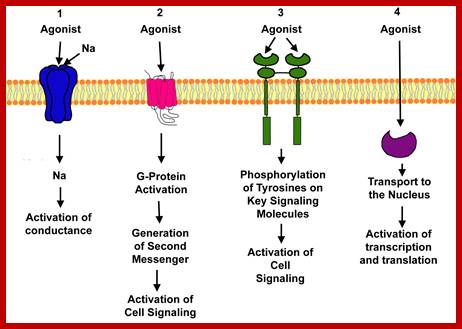
Drugs that interact can be agonists and antagonists; Ions channels Ligand gated, voltage gated ,second messengers, G protein coupled, receptor protein kinase, and Intercellular hormone receptors. http://www.uky.edu
Receptor Tyrosine Kinases:
They form an important class of receptor kinases –enzyme linked cell surface proteins. The ligands are soluble molecule or membrane bound components. The ligands are many such as insulin, EGFs, PDGF and several growth factors. The receptor protein consists of a single transmembrane pass domain; the receptor does not get activated with the binding of the ligand to single receptor proteins; the binding of the ligands makes the receptors dimers, which makes the receptor active and start phosphorylating tyrosine residue at cytosolic sites (autophosphorylation). Most of these receptors require dimerization to be active. Intracellular signaling proteins that bind to RTKs in cytosol get activated by phosphorylated tyrosine moiety.
http://www.motifolio.com/
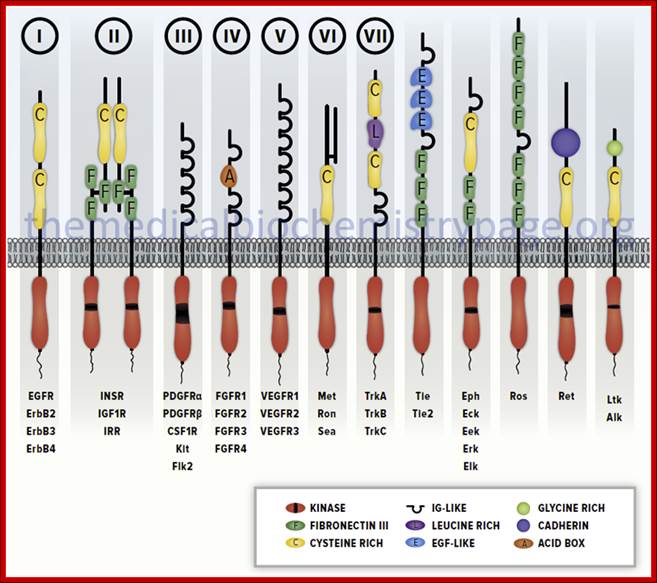
Diagrammatic representation of several members of the receptor tyrosine kinase (RTK) family. Several members of each recepetor sub-family are indicated below each representative. The Roman numerals above the first seven sub-types correspond to those sub-types described in the Table above. These RTK sub-types do not represent the entire RTK family sub-types.http://themedicalbiochemistrypage.org/
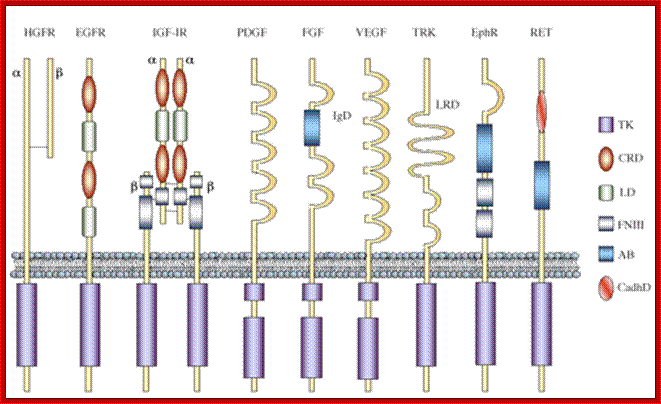
Schematic structure of the main human RTK families. Abbreviations are: TK, tyrosine kinase domain; CRD, cystein-rich domain; LD, leucine domain; FNIII, fibronectin type III-like domain; AB, acidic box; CadhD; cadherin-like domain; LRD, leucine-rich domain; IgD, immunoglubulin-like domain.Receptor protein kinases; Francesca De Bacco, Michela Fassetta and Andrea Rasolahttp://www.cancer-therapy.org/
http://bloodjournal.hematologylibrary.org/
Ligand binding leads to dimerization-http://oregonstate.edu/
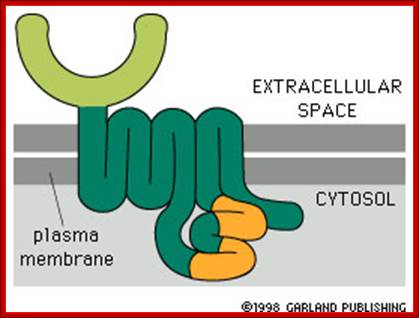
http://www.bio.miami.edu/
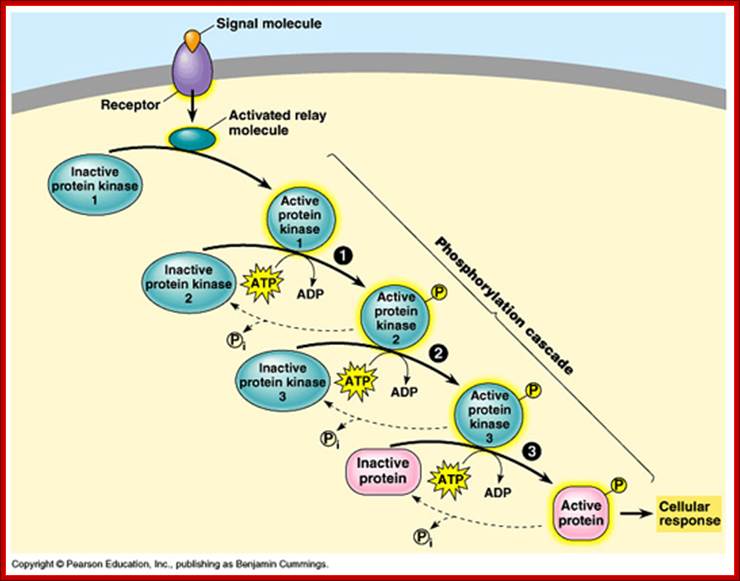
Activation and phosphor relay. https://apbiologywiki.wikispaces.com
Once they are phosphorylated they activate other cytosolic enzymes by their kinase activity, the product of the kinase cascade called MAPK moves into the nucleus and activate TF by phosphorylation which bind to promoter elements to activate certain genes. For example NFkB remains inactive in cytosol by an inhibitor-I. Phosphorylation of the inhibitor frees the NFkB to move into the nucleus to activate few genes. In general signal cascade initiated by RTKs begins with small monomeric G-proteins called RAS.
RTK- Ras pathway:
Receptor Ras belongs to a super family of monomeric G-proteins related to another super family of heterotrimeric G-proteins, especially G-alpha. RAS members belong to a family of monomeric proteins such as Raf, Rab, CDC4 and Rho/Rac; all belong to the RAS super family.
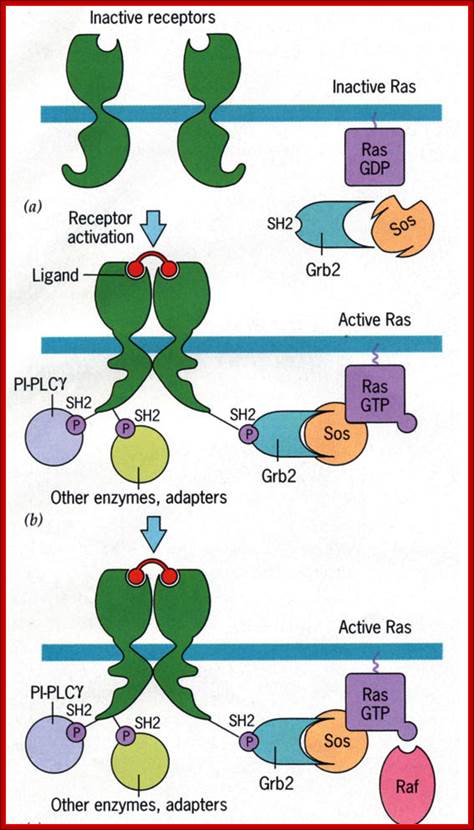
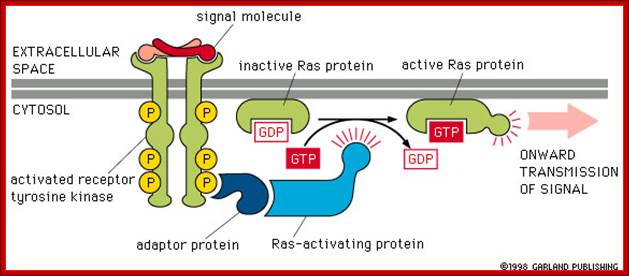
http://www.bio.miami.edu
Rho and Rac relay signaling from surface receptors to actin
cytoskeletons; and the members of Rab family of GTPases which are involved in trafficking intracellular proteins, but the Ras members which are located at the inner surface of plasma membranes initiate a kinase cascade which has tremendous effect on cellular metabolism as well as transcriptional activation of genes.
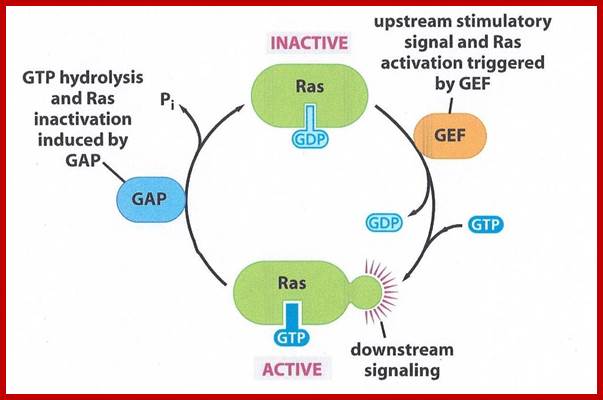
GTPase and Ras; https://biology-forums.com
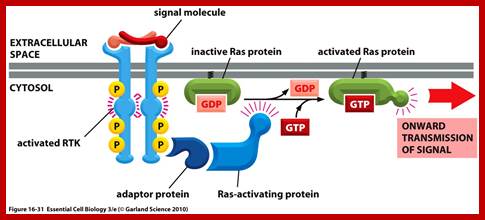
G. linked receptor kinases; http://oregonstate.edu
Ras gene was originally discovered as an oncogene causing cancer and found to be a normal cellular gene, but mutated. Ras is GTP binding monomeric protein (21kd) exists in Ras-GDP inactive and Ras-GTP active form. Ras itself as GTPase activity, converting GTP to GDP, it becomes inactive. Mutant Ras cannot hydrolyze GTP, thus remains active all the time. Ras members consists of 60 or so distinct members in mammalian systems, divided in to Ras, Rab, Rho, Arf, Ran and Gem subfamilies. In fact, plants like Arabidopsis contains 93 genes encoding GTP binding proteins (plants have all the said subfamily members but not Ras).
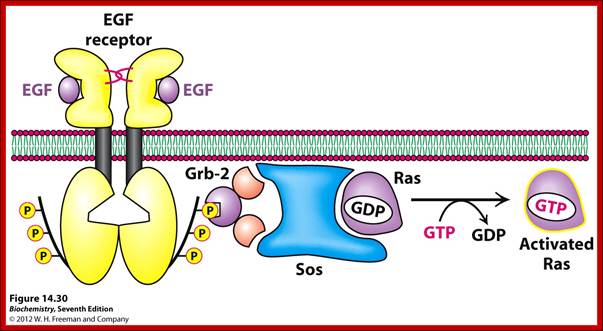
Activation of RTKs initiates with the binding of ligands and dimerization of RTK and autophosphorylation of its own cytosolic domain. Phosphorylation of receptor causes the binding of Grb2 protein which is also associated with Sos (son of seven). Thus Grbs-Sos binds to the p-lated RTK. The SOS then binds to inactive Ras which is anchored to the inner surface of the membrane. Binding of SOS to Ras leads to the release of GDP and the binding of GTP, thus Ras becomes active. The Ras now provides the binding sites for Raf, which is serine/threonine kinase; this initiates a chain of events while Ras is tyrosine kinase. Many growth hormones such as EGF binding to EGF receptor can lead to Ras independent pathway of activation. For example EGF, in C.elegans, controls contraction of smooth muscle cells. Such EGF, non RAS pathway, regulates the extrusion of oocytes from one compartment of bisexual gonads to the other compartment where they are fertilized. Coupling of EGF receptor to RAS is not actually required for EHF induced muscle contraction in many systems.
GRB2 consists of two domains one called and SH2 and the other SH3. SH stands for Src homology. These protein domains are protein-protein interacting domains. The SH2 domain has a central antiparllel sheets flanked by 2 alpha helices. The target for this binding is a three a.a sequence pYEET where tyrosine phosphorylated. Similarly the GRB2 contains another domain called SH3, which is also protein- protein interacting domain, consists of 8-10 a.a sequence RXLPPLLXY or XXXPPLPXR. Thus GRB2 interacts with p-lated tyrosine in the cytosolic receptor domain and its end interacts with SOS which to has proline rich pocket for bind of GRB2. GRB2 acts as an adopter protein between RTK Receptor and SOS-Ras. Proteins do contain many such protein-protein interacting domains such as PH (pleckrin homology), PID phospho tyrosine interacting domain (NPXPY).
The p-lated tyrosine binding to GRB2-SOS interacts with Ras-GDP, which is associated with inner surface of the membrane, thus induce RAS to exchange GDP with GTP (assisted by GTP exchange factor GEF), thus Ras become active.
Ras is a monomeric G-protein a tyrosine kinase. Activated RAS-GTP transfers its Phosphorous to another accessory protein called Raf; this GTPase activity is assisted by GTPase called GAP. Ras contains about 170a.a and G-alpha contains 300a.a. yet their 3-D structure is similar and both belong to super family GTPase proteins.
Ras plays a crucial role in activating cell cycle events, if there is a mutation at 12th glycine it block the GTPase activity and RAS bound to GTP remains active for a long time; this can lead to cell proliferation and cancer. Mal function of RAS receptor can lead to cancer. Malfunction of GEF can lead to cancer. Malfunction of GAP can lead to cancer. Ras malfunction can lead to cancer, malfunctioning of it receptor leads to cancer, malfunctioning of its associated GEF and GAP leads to cancer. Mutation in Ras at 12 a.a glycine position renders Ras active all the time for the bound GTP remain bound for a long time; thus it is found to induce cancer; for that matter a majority of cancer types contain mutated Ras.
Raf leads to a sequence of events one leading to the other like a cascade. Raf is considered as MAP (mitogen activated protein kinase kinase kinase, MAPKKK); MAPKKK phosphorylates MAPKK which then phosphorylates MAP kinase (MAPK). Active Ras p-lates Raf which is serine/threonine kinase. Raf p-lates MEK (MEK is Tyrosine/serine/threonine kinase) and MEK-p plates MAPK (MAPK is serine/threonine kinase); this is a kinase cascade, ultimately the phosphorylation cascade ends in the nucleus; by the time the cascade reached the nucleus, it activates many other proteins laterally.
MAP/ERK kinase pathway:
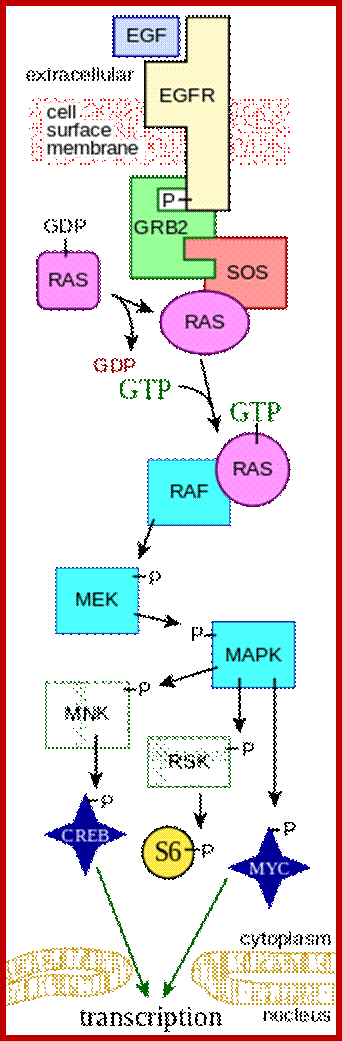
Key components of the MAPK/ERK pathway. "P" represents phosphate, which communicates the signal. Top, epidermal growth factor (EGF) binds to the EGF receptor (EGFR) in the cell membrane, starting the cascade of signals. Further downstream, phosphate signal activates MAPK (also known as ERK). Bottom, signal enters the cell nucleus and causes transcription of DNA, which is then expressed as protein. https://en.wikipedia.org; https://www.researchgate.net
In both cytokine receptor and RTK receptors signal transduction takes place via GRB2, SOS and Ras. Activated Ras promotes three sequentially acting protein kinase steps. The kinases are associated with a scaffold region. This cascade of activation of phosphorylation ends in MAP kinase, which is a serine/threonine kinase also known as ERK for this refers to external signal cascade. Activation of MAPK in different cell types can lead to similar or different responses.
Ras-Raf signal cascade:
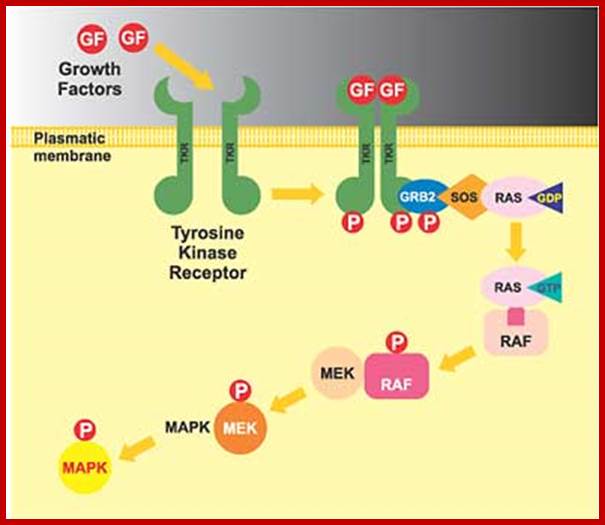
Sorbera, L.A., Castaner, J., Bozzo, J., Leeson, P.A.https://journals.prous.com
The Ras/Raf/MEK pathway is a signaling module that controls cell growth and survival. Activation of this pathway results in a cascade of events from the cell surface to the nucleus ultimately affecting cellular proliferation, apoptosis, differentiation and transformation. Raf is a serine/threonine kinase that is a downstream effector enzyme of Ras. When activated, Raf goes on to activate MEK1 and MEK2 kinases which in turn phosphorylate and activate ERK1 and ERK2 which translocate to the nucleus where they stimulate pathways required for translation initiation and transcription activation leading to proliferation. Raf kinase has been validated as a potential and attractive target for hyperproliferative disorders such as cancer. Research has recently focused on efforts to discover potent Raf kinase inhibitors and several low-molecular-weight Raf kinase inhibitors have been described. Bis-aryl ureas were identified within this program using medicinal chemistry-directed syntheses or combinatorial libraries. After high-throughput screening of more than 200,000 compounds against recombinant Raf-1 kinase, the orally active Bay-43-9006 was identified as having potent inhibitory activity and was chosen for further development as a treatment for cancer. Bay-43-9006 has exhibited potent in vitro activity against several tumor cell lines and has displayed efficacy in human tumor xenograft models. Moreover, results from phase I development in patients with a variety of cancer types indicates promising clinical efficacy for the compound.
Activated Ras which is bound by GTP binds to N-end of Raf (which is serine/threonine kinase). Hydrolysis of GTP releases Raf-GTP (active). Raf-GTP activates MEK, stands for MAPK, ERK kinase, (again by p-lation at serine threonine residues). MEK (tyrosine kinase) P-lates MAPK. MAPK (serine/threonine kinase) p-lates many other proteins including transcriptional factors. Mutated Raf with missing N-end can also induce cancer.
EGF, PDGF and few other growth hormones induce cells at Go to express more than 100 or more genes called early genes, to be induced before the cell enters into S-phase. One early response gene expressed is Fos gene. With other gene products such as Jun, Fos induces the expression genes required for progression through cell cycle. Majority of growth factors that stimulate transduction through RTKs induce cFos and few other similar genes. These genes have enhancer elements for growth factors like SRE (serum response elements). Many TF bind to such elements and some of them are activated by MAPK. It also activates a cytosolic kinase called c90 RSK, which translocates into the nucleus, where it p-lates specific serine residue in SRFs. MAPK a dimeric protein, also p-lates serine in TCF, which associates with two other p-lated SRFs, thus a trimeric complex binds to SRE elements of cFos gene. Some of the genes expressed are found to be TFs which in turn activate other required genes for cell proliferation.
For example one of the genes stimulated by MAPK is Fos, it is a proto-oncogene; can cause cancer. When the Fos protein combines with phosphorylated Jun, one of the nuclear proteins that is p-lated by MAPK, forms heterodimers and binds to gene regulatory element called AP1 and turns on the genes. The genes such as MYB and Myc are turned on by MAPK phosphorylation of TFs. In plants phytochrome, Gibberellins and other plant hormones work through such phosphorylation cascade and regulate gene expression by turning on MYB like TFs.
Though it is well recorded that RTKs generally produce a cascade effects ending is MAPK, several of trimeric G proteins too also induce signaling in MAPKs. E.g. Yeast mating pathway. Yeast and many other unicellular systems lack cytokine receptor or RTK pathways, yet they exhibit several alternative MAPK pathways.
GPCR and RTK Combined Activity:
Role and action of Yeast mating factors:
Even though each of the said GPCR and RTK receptor induced cascade is prevalent in many system one cannot exclude the cross activity of both, which both ultimately lead to nuclear transcriptional activities.
https://biologyhealth.com
Yeast mating factors is a paradigm for genetic transduction pathway in reproduction between two opposite strains. Mating factors secreted by yeast cells are ‘a’ and ‘alpha’. Haploid cells ‘a” secretes ‘a’ but contains ‘alpha ‘receptors. ‘Alpha cells secrete ‘alpha’ factors but they have ‘a’ receptors. Factor ‘a” bind to ‘alpha ‘receptors and ‘alpha’ factors bind to ‘alpha’ receptors. Activation of these receptor induce signal pathway leading to MAPK and lead to the expression of genes that inhibit progression into cell cycle and thus opposite mating types fuse to form 2n cells.
Ligand binding to these receptors, which are trimeric G proteins trigger GTP binding and activation where G-alpha-GTP dissociate from beta/gamma dimers. https://www.researchgate.net
This is similar to that of GPCR system, where induced G-alpha dissociates and activates signaling pathway. In yeast cells all physiological responses are mediated by yeast pheromones which act on G-bg. In yeast freed Gbg (beta and gamma subunits), triggers a kinase cascade analogous to Ras-Raf pathways. Analysis of ‘a’ and ‘alpha’ receptors and G-proteins, mutants show they contain ‘a’ and ‘alpha’ functional G-receptors, yet sterile and defective mating habits. In such cells G.bg are tethered to membranes via gamma subunit and activates Ste20 a protein kinase. It activates Ste 11; it is a serine/threonine kinase analogous to Raf or MEKK. Activated Ste11 plates and activates Fus3, which is ser/threonine kinase equivalent to MAPK. After translocation into the nucleus, Fus3 promotes expression of target genes by the p-lation of TFs such as Ste12. which is responsible for expression of mating factors? The other factors in yeast mating events are Ste5, which interacts with G-bg, Ste11, Ste7, Fus3, Ste5; they all act as scaffold for the assembly for other components becomes active G-alpha-GTP and G-bg dissociate but still held on to the membrane via gamma subunit. It provides a scaffold for the binding and activation of several factors such as Ste20 (ser/thr), Ste11 (mEKK, ser/thr), Ste7 (mek ser/thr), Fus3 (MAPK ser/thr), Ste12 (TF) in the given order or sequence. In these events the Sate5 provides scaffold for the assembly of all the above said complexes.
Note all MAPK kinase activation requires p-lation at Tyrosine/Thr at the lip region. MEK is a dual kinase (belong to another super family of proteins) plates several members of MAPK family. All signals end in one way or the other in MAPK as the end of the kinase cascade. Such system has been found in humans, drosophila and yeast. Specific kinases are activated in response to signals such as pheromones, starvation, high osmomolarity, hypotonic shock and carbon-nitrogen starvation. In both and higher EKs different MAPKs cascade share a cascade of common components.
|
Signal |
kinase |
response |
|
pheromones |
Fus3 |
mating |
|
starvation |
KSS1 |
filamentous |
|
High osmomolarity |
Hog1 |
Osmolyte synthesis |
|
Hypotonic shock |
MPK1 |
Cell wall dissolving |
|
Carbon. Nitrogen starvation |
Smk1 |
sporulation |
|
|
|
|
TGF beta Signal Transduction:
Transforming growth factor beta is an excellent example to illustrate its structure and action. TGFbeta belongs to a super family of ligands; one of them is bone morphogenic protein (BMP) which induces bone formation under in vitro cell culture. Presently BMP7 is used to strengthen the fractured bones. There are several BMP; some of them help in developing pathways such as development of mesoderm and blood forming cells. TGFbeta acts as a proliferative component in many mammalian cell types. TGF b also signals overcome TGFb induced growth inhibition. In Drosophila an equivalent of TGFb is DPP which controls dorso-ventral pattern formation. Though there are a variety of TGFs, once they bind to the ligands downstream activity is more or less same.
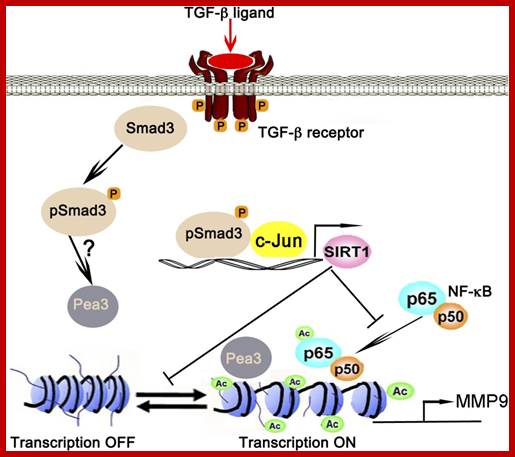
TGF-β-Smad3 signaling in emphysema and pulmonary fibrosis: an epigenetic aberration of normal development? Epigenetic regulation of MMP9 by TGF-β-Smad3 signaling: TGF-β ligand activates TGF-β receptor-Smad3 signaling, which in turn collaboratively activates SIRT1 transcription with c-Jun. SIRT1 in turn deacetylates NF-κB at lysine 30 and histones at the transcription factor AP-1, NF-κB, and Pea3 binding sites of MMP9 promoter to suppresses its transcription, thereby fixing MMP9 in the OFF mode; conversely when Smad3 is missing MMP9 stays in the ON mode. Ac, acetylations. , , http://ajplung.physiology.org
TGF beta as a ligand; http://www-nmr.cabm.rutgers.edu
Each of the TGFb is synthesized as precursor proteins with prodomain and processed into mature protein (mature domain). Secreted TGFb remains in ECM as inactive complex, for it is bound to latent TGFb binding protein (LTBP). A matrix protein like thromboprotein or integrin binding to the inactive TGFb induces a change in conformation as an active form. TGFb is 110-140a.a long has four antiparallel strands and 3 S-S bonds, such internal molecular bonds form what is called as cysteine knots, resistant to denaturation. An additional N-terminal S-S bond between two monomeric proteins makes it dimer. Binding of the ligand to its receptor has been demonstrated with FRET technology.
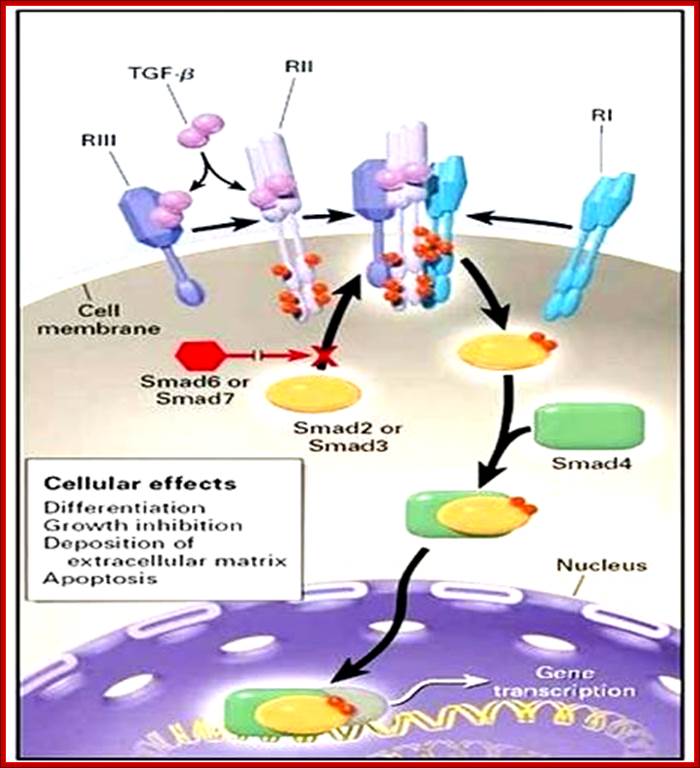
The receptor sits on the surface of the cell. The receptor proteins purified show proteins with different mol.wt 55, 85 and 280 Kda called R-1, R-2, and R-3. Among them R3 is the most abundant protein, it is called cell surface proteoglycan. It binds to TGFb and concentrates at cell surface. Type I and II are dimeric transmembrane receptor proteins with serine/threonine kinase activity at their cytosolic domain. R-II performs autocatalysis. Binding of the ligand induces formation of complexes containing each of the R1 and R-II, thus active RI kinase activity at the cytosolic domain. Phosphorylated domain interacts with cytosolic proteins such as Mads. There are three different kinds Mads proteins such as R-smad1, co sMads, and inhibitor sMads. Only sMads that is p-lated enters the nucleus along with smad-4. R-sMads consists of two domains called MH1 and MH2 separated by a flexible linker. The N-end of MH1 contain nuclear localization signals (NLS) and a DNA binding motif. The R-S mads contain two motifs one NLS and another DNA bonding motifs. When the smad is not p-lated its NLS is masked by the MH2 or called DNA binding motif. When R-sMads get p-lated at MH2 they open up and associate with importin-b, smad3 and smad 4 enter into the nucleus as a complex of 2smad-3 proteins and one smad-4 (co-smad) unit. Once they enter into the nucleus, they dissociate from b-importin and bind to DNA regulatory elements. They also get associated with other TFs and form a gene activation complex. Such activation complex, at plasminogen activator gene, activates an inhibitor gene called PA1-1. In the nucleus when the sMads are dephosphorylated they are exported back to the cytosol as dissociated subunits. So the concentration of active sMads depends on the level of active TGFb.
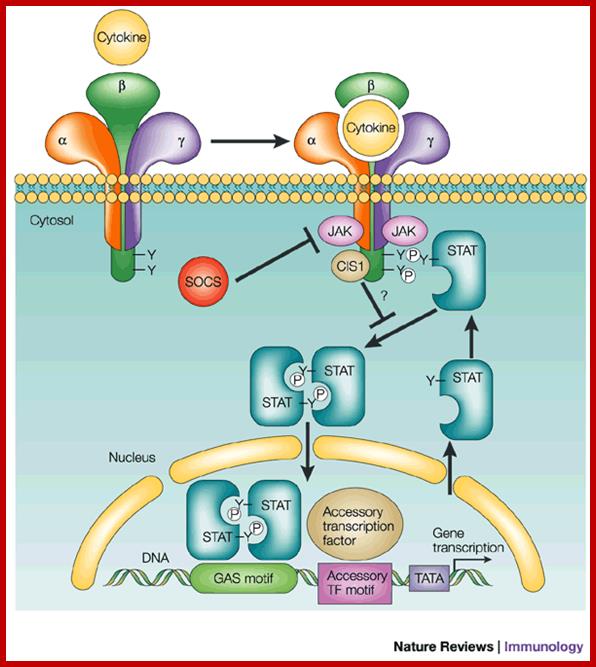
Cytokine signal transduction:
Interferons and cytokines are synthesized in response to infection at surfaces as the first line of defense what is called as innate immunity. Cytokines are a family of cellular secreted products that act as defense structure against viruses and bacteria within 12 to 24 hrs of infection. Cytokines induce signal transduction via specific cell surface receptors. The receptors bind to their respective cytokines and respond and transduce cellular responses. Receptors consist of extracellular domains, where they bind to ligands and a transmembrane domain and an extended cytoplasmic domain. A single ligand binding can induce dimerization and activation of kinase activity. Receptor proteins in a dimer can vary from one monomer to the other; for one of the monomer may contain JAK kinase activity at its cytosolic side and another receptor protein may possesses Tyrosine kinase activity. Both cytokine and RTK receptors once activated go through autophosphorylation (after dimerization) and act as binding or docking sites for proteins with SH2 domain such as IRS1 or multi docking protein via PTB domain.

Domain structure of Class I cytokine receptors;
Domain structures depicted for the homomeric signalling receptors, and examples of heteromeric receptors that interact with the shared γc, gp130, βc and LIF-R (LIFR).
;http://www.biochemj.org
Cytokines form a family of small secreted proteins of 160 a.a long. They control growth and differentiation of specific cell types. Prolactin, at the time of pregnancy, induces epithelial cells of immature ductless in the mammary gland into acinar cells that produces milk and secrete into the ducts. IL2 is required for the formation and function of T-cells. IL4 is required for growth and function of B-cells. Interferon alpha another form of cytokine, induced in response to viral infection of cells, once secreted acts on neighboring cells to become active and render them in anti viral state. Another cytokine called erythropoietin (epo) triggers the development of RBC from erythrocyte progenitor cell types in bone marrow. Erythropoietin is synthesized in kidney cells (which monitor the level of oxygen in the blood). Low oxygen induces oxygen sensitive TF called HIF1alpha to synthesize more erythropoietin and secrete into the blood. With this more progenitors are saved from death, allowing each cell to generate 50 or more RBC cells in a period 2 days. This way the body responds the loss of blood. Many cytokines induce differentiation of different types of blood cells, such as Granulocytes. Granulocyte colony stimulating factor (G-SF) induces specific progenitor in bone marrow to divide and differentiate into granulocyte s which is a kind of WBC that inactivate bacteria, virus and other pathogens. In cancer therapy specific GSFs are administered to stimulate proliferation and differentiation of granulocytes that leads to platelets formation required for clotting.
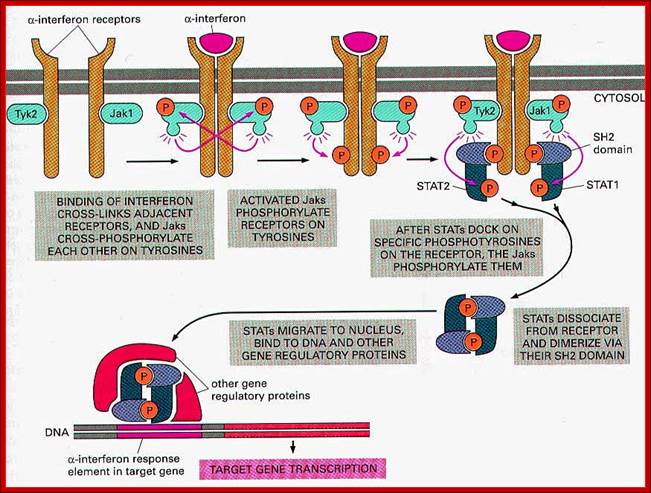
In this schematic, the receptor is a
three-subunit receptor (![]() ,
, ![]() and
and ![]() ); for example,
interleukin (IL)-2 or IL-15. Many other cytokines have receptors that are
homodimers or heterodimers. Binding of the cytokine induces the activation of
JAKs (Janus-activated kinases), which then can phosphorylate cellular
substrates, typically including at least one of the receptor chains. This
allows the recruitment of STAT (signal transducer and activator of transcription)
proteins to the phosphorylated receptor by their Src-homology 2 (SH2) domains,
which, in turn, are also phosphorylated. The STAT proteins can dimerize,
translocate to the nucleus and bind DNA. The TATA box allows the binding of
basal transcription machinery by the transcription factor TF-IID. The
'accessory' transcription factor is shown to indicate that STAT proteins do not
necessarily act alone, but that STAT-dependent responses can be differentially
influenced by other transcription factors. Shown are SOCS (suppressor of
cytokine signalling) family proteins, denoted as SOCS and CIS1
(cytokine-inducible SH2-containing protein 1), which can negatively regulate
JAK/STAT signalling. CIS1 is shown as bound to the receptor, inhibiting STAT
activation, and SOCS is shown as a JAK inhibitor36, 37. GAS motif,
); for example,
interleukin (IL)-2 or IL-15. Many other cytokines have receptors that are
homodimers or heterodimers. Binding of the cytokine induces the activation of
JAKs (Janus-activated kinases), which then can phosphorylate cellular
substrates, typically including at least one of the receptor chains. This
allows the recruitment of STAT (signal transducer and activator of transcription)
proteins to the phosphorylated receptor by their Src-homology 2 (SH2) domains,
which, in turn, are also phosphorylated. The STAT proteins can dimerize,
translocate to the nucleus and bind DNA. The TATA box allows the binding of
basal transcription machinery by the transcription factor TF-IID. The
'accessory' transcription factor is shown to indicate that STAT proteins do not
necessarily act alone, but that STAT-dependent responses can be differentially
influenced by other transcription factors. Shown are SOCS (suppressor of
cytokine signalling) family proteins, denoted as SOCS and CIS1
(cytokine-inducible SH2-containing protein 1), which can negatively regulate
JAK/STAT signalling. CIS1 is shown as bound to the receptor, inhibiting STAT
activation, and SOCS is shown as a JAK inhibitor36, 37. GAS motif, ![]() -interferon activated
sequences (see Box 2); P, phosphorylated tyrosine (Y) .
-interferon activated
sequences (see Box 2); P, phosphorylated tyrosine (Y) .
Signalling by the JAK/STAT pathway for a typical type I cytokine; Warren J. Leonard; http://www.nature.com
Almost all cytokine receptors contain similar tertiary structures in having 4 long helices folded into specific module. Each of them contains two extracellular sub domains containing beta strands folded together. In the case of erythropoietin receptor binding of the ligand to the receptor (single ligand binding to two receptor monomers) is typical of cytokine receptors (dimers).
Cells express specific receptor; the number and kind vary from one cell type to the other. Such cells respond to specific kind of cytokines depending upon the kind of TFs, chromatin nature and other proteins. Intracellular signaling pathways to different kinds of ligands and receptors are more or less similar. All eventually lead to the activation of TFs and gene expression.
For example Epo-EpoR and JAK2 pathway responds to Epo, binds to Epo-Receptor which activates JAK2 pathway. This pathway acts and uses STATs for transcriptional activation. This path way also activates RAS pathway through GRB2; with a cascade of kinase –kinase activity, activate MAPK kinase that activates several TFS; Activated JAK2 pathway and also activate Phospholipase-C. this can lead to rise in ca+ ion level which has tremendous effect on cellular activity including gene expression. The JAK2 also activates PI3 kinase which in turn activates a string of responses including activation of TFs.
JAK2 kinase and STAT pathway:

Polycythemia Vera;http://flipper.diff.org
The cytokine receptors containing JAK2 activity have an exoplasmic ligand binding domain, a membrane pass domain and a cytosolic domain with kinase activity. The activated receptors having JAK kinase become dimmers and p-late their own Tyrosine residues. Tyrosine P-lated sites become the binding sites for SH2 domain containing STATs (stimulation of transcription and activation of transcription), which are a group of TFs. All STAT proteins contain an N-terminal SH2 domain, a central DNA binding domain and a tyrosine residue at C-end of the protein. Activated JAK2 p-lates tyrosine residues in STAT at C terminal. The p-lated STATs dissociate from the receptors and dimerize, where each p-lated SH2 domain binds p-lated site. Dimerization exposes NLS site for the import of the same into the nucleus. The STATs (they form different combination of STATS in different cell types) and bind specific enhancer region and activate gene expression. Different combination of STATs expresses different genes. In erythroid progenitors STATs express BCLX gene, which prevent apoptosis and allow cell proliferation. Mice lacking STATs suffer from genetic anemia, yet mice survive but weak.
The downstream activation of Epo-EpoR-Jak2 pathway is multifold. The cytosolic p-lated domain binds with an adapter protein GRB2, which reacts with Ras. Ras activates MAP kinase that leads to activation of Transcription. This also activates Phospholipase-C which results in opening of Ca= channels and calcium level increase. This can lead to regulation of gene expression. JAK2 also activates PI3 kinase, which activates proteinase B which can lead to activation or repression of few cellular proteins.
Cytokine signals are modulated in the sense STATs activated transcriptional activity is time bound, not too long nor too short. This is regulated by SHp1 phosphotase. Mutant mice for SHp1 die because of excess production of erythrocytes and other cells. SHp1 negatively regulates several kinds of cytokine receptors in progenitor cells. In the case of Epo stimulated cells, receptor bound JAK kinase2 is dephosphorylated by SHp1, which has p-tyrosine binding domain at one side and at the end it contains phosphotase domain. Use of these domains the JAK kinase is regulated.
{ell Surface and Cytosol Receptor:
A large number of cell surface protein receptors act as transducers, enzyme themselves or associated with enzymes and receptor channels. These receptors consist of exoplasmic ligand binding domain, a single pass transmembrane (helix) domain and an elaborate cytosolic domain which extends deep into cytosol. They don’t interact with trimeric G-proteins. These are six (mainly) classes of receptors in animal systems; Receptor tyrosine kinases (RTK), Receptor tyrosine phosphotases, Receptor serine/threonine kinases, Tyrosine kinases linked- receptors, Receptor guanylate cyclases, and surface proteases. Among them RTKs and Receptor serine threonine kinases (RSK) are more abundant, similar type of receptors are also found in plants called Plant receptor kinases (PRKs). Whether the receptor is GPCR like or RTK like, all of them as they are anchored in membranes, they are always in motion laterally, because the membrane components are fluid like and in constant movement. Often receptors are endocytosed and kept inside the cytosol and when required they are moved back to the cell surface, e.g. Insulin receptor. In many cases where the signal transduction to be downgraded membrane receptors are endocytosed and degraded.
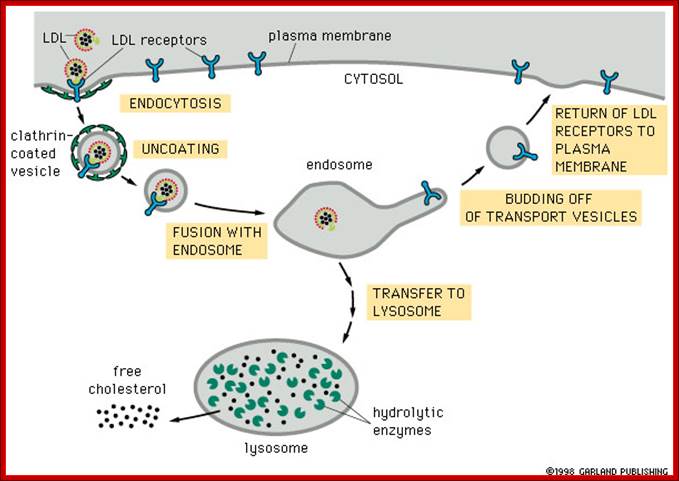
https://www.studyblue.com
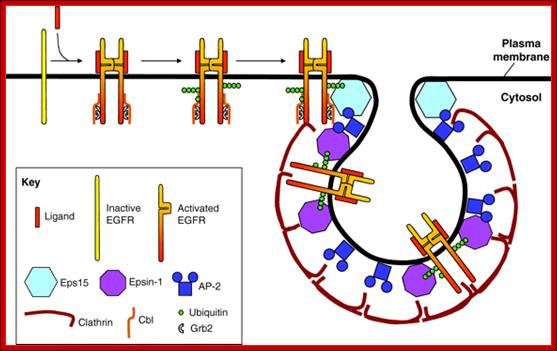
Current model illustrating the events required for recruitment of EGFR into clathrin-coated pits. We propose the following scenario for ligand-induced clathrin-dependent EGFR endocytosis: ligand binding to EGFR induces receptor dimerization and kinase activity. Kinase activity results in the phosphorylation of tyrosines in the EGFR tail, allowing Cbl to bind directly, or indirectly through Grb2. Recruitment of Cbl results in mono- and polyubiquitylation of the EGFR tail. Appended polyUb chains interact with Ub-interaction motifs (UIMs) of epsin-1 and Eps15. Because Eps15 is localized at the rim of clathrin-coated pits (Stang et al., 2004; Tebar et al., 1996), we propose that ubiquitylated EGFR initially interacts with Eps15. In contrast to Eps15, epsin-1 is localized all along the clathrin coat; we further propose that EGFR is transferred to epsin-1 and thereby recruited into the central region of clathrin-coated pits (Kazazic et al., 2009). The clathrin-coated membrane subsequently invaginates and is released into the cell interior as a clathrin-coated vesicle. Internalization and intracellular sorting of the EGF receptor: a model for understanding the mechanisms of receptor trafficking; Inger Helene , adshus1,2,* and Espen Stang1; http://jcs.biologists.org/ http://jcs.biologists.org
Receptor Tyrosine Kinases:
They form an important class of receptor kinases –enzyme linked cell surface proteins. The ligands are soluble molecule or membrane bound components. The ligands are many such as insulin, EGFs, PDGF and several growth factors. The receptor protein consists of a single transmembrane pass domain; the receptor does not get activated with the binding of the ligand to single receptor proteins; the binding of the ligands makes the receptors dimers, which makes the receptor active and start phosphorylating tyrosine residue at cytosolic sites (autophosphorylation). Most of these of receptors require dimerization to be active. Intracellular signaling proteins that bind to RTKs in cytosol get activated by phosphorylated tyrosine moiety.

Mechanism of action of tyrosine kinase. 1. Receptor expression at membrane claveola 2. Ligand binding 3. Hetero/homodimerization leading to tyrosine kinase activation and tyrosine transphosphorylation 4. Signal transduction 5. Receptor internalization 6. Receptor degradation or re-expression; http://www.medsci.org/
Insulin like growth factor receptors in Autocrine growth loop;
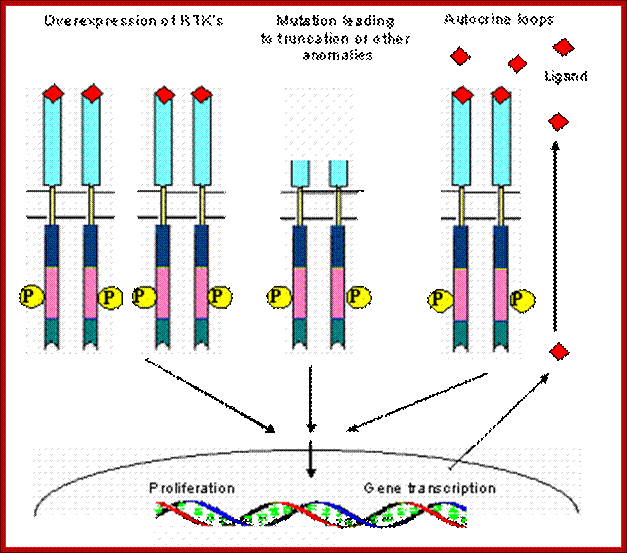
Coexpression of IGFR and its ligand IGF I and IGF II is reported in the pathogenesis of breast cancer, prostrate cancer and small cell lung cancer . Elevated IGF-I R autophosphorylation and kinase activity has been reported in breast cancer [28] . Elevated prostrate cancer risk is also correlated with elevated plasma IGF-I levels [29] . Hence a strong association of autocrine paracrine loops has been implicated in the pathogenesis of several cancers.Schematic view of different mechanisms leading to the constitutive activation of tyrosine kinas ; Manash K. Paul , Anup K. Mukhopadhyayhttp://www.medsci.org/

The epidermal growth factor receptor signalling network. The discovery of receptor tyrosine kinases: targets for cancer therapy;
a | Ligand binding to the epidermal growth
factor receptor (EGFR) induces dimerization through a receptor-mediated
mechanism. Signal diversification is generated by the presence of multiple
EGF-like ligands and the formation of different dimeric receptor combinations. b | Receptor dimerization results in
cross-autophosphorylation of key tyrosine residues in the cytoplasmic domain,
which function as docking sites for downstream signal transducers. EGFR
stimulation results in activation of signalling cascades that include the
RTK–GRB2–SOS–RAS–RAF–MEK–ERK, PI3K–AKT, PLC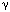 and STAT pathways.
EGFR can activate PI3K through RAS-GTP in some cell types. c | EGFR acts as a point of convergence for heterologous
signals from G-protein-coupled receptors (GPCRs; metalloprotease-mediated EGFR
signal transactivation), cytokine receptors, integrins, membrane depolarization
and agents that are induced by cellular stress. The EGFR thereby defines
crucial cellular responses, such as proliferation, differentiation, motility
and survival. ERK, extracellular-signal-regulated kinase; GEF, guanine-nucleotide-exchange
factor; PI3K, phosphatidylinositol 3-kinase; PLC
and STAT pathways.
EGFR can activate PI3K through RAS-GTP in some cell types. c | EGFR acts as a point of convergence for heterologous
signals from G-protein-coupled receptors (GPCRs; metalloprotease-mediated EGFR
signal transactivation), cytokine receptors, integrins, membrane depolarization
and agents that are induced by cellular stress. The EGFR thereby defines
crucial cellular responses, such as proliferation, differentiation, motility
and survival. ERK, extracellular-signal-regulated kinase; GEF, guanine-nucleotide-exchange
factor; PI3K, phosphatidylinositol 3-kinase; PLC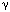 , phospholipase C
, phospholipase C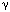 ; STAT, signal transducer and activator of transcription. Andreas
Gschwind, Oliver M. Fischer & Axel Ullrich; http://www.nature.com/
; STAT, signal transducer and activator of transcription. Andreas
Gschwind, Oliver M. Fischer & Axel Ullrich; http://www.nature.com/
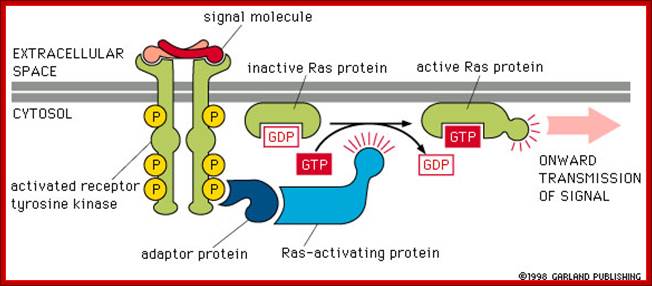
These kinases activate an important GTP binding protein called Ras.(look) Ras activation leads to a cascade of activation of kinases and eventual changes in gene regulatory proteins. Gene transcription and thus gene expression are changed, causing changes in cell proliferation and cell differentiation; http://www.bio.miami.edu/
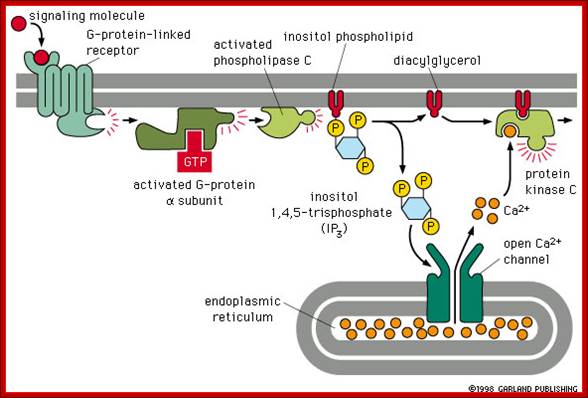
Other hormones use the inositol phosphate pathway (phospholipase C activation). Examples of these hormones are vasopressin and thrombin. Acetylcholine, a common neurotransmitter, also uses the inositol phosphate pathway. (Do you remember that we noted in a previous lecture that inositol phospholipids were found as part of the the internal layer of the lipid bilayer membrane?) (look) The inositol pathway is involved with release of Ca++ from the ER. This release is involved in many important biological processes, including fertilization. Look at this beautiful picture to see what happens when a starfish egg is fertilized. A Ca++ sensitive fluorescent dye records rising concentrations of Ca++, .As we shall see, it is Ca++release from sarcoplasmic reticulum in muscle which activates skeletal muscle contraction. (Do you now know which hormones use the cAMP or the inositol phosphate pathways? Do you know what becomes of the inositol phospholipids?),http://www.bio.miami.edu/
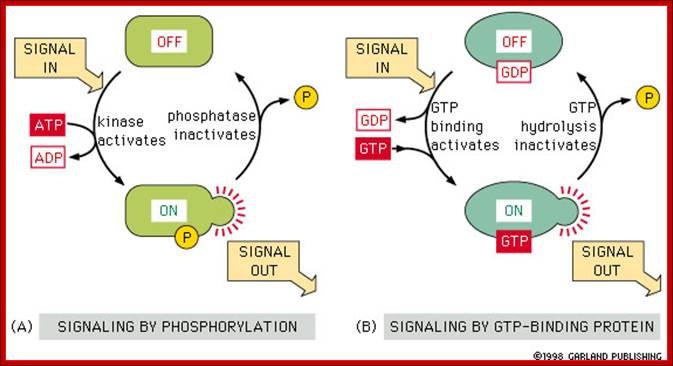
Other external cell signals, use one of three mechanisms to pass the "message" into the cell interior - a) An ion channel linked receptor, b) a G-protein linked receptor, c) an enzyme linked receptor. (look)
· G protein receptors are a major pathway for converting an external signal into an intracellular message. (look)
· G proteins consist of three subunits. The large alpha unit dissociates from the rest of the molecule and diffuses along the internal cell surface when a signaling molecule binds to the external receptor binding site. (look) This dissociation is accompanied by release of GDP and binding of GTP. Binding of GTP is common in molecular switches. (look)
· G proteins cause K+ channels to open in response to neurotransmitter binding in heart muscle. (look); ;http://www.bio.miami.edu/
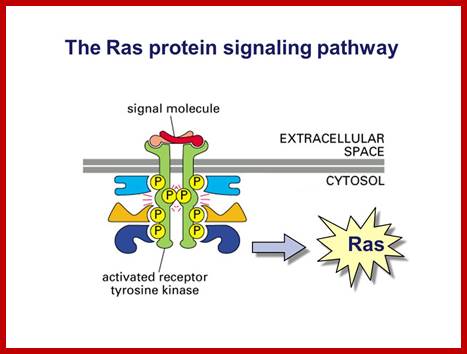
Ras protein signalling pathway; www.slidePlayer.com
Once they are phosphorylated they activate other cytosolic enzymes by their kinase activity, the product of the kinase cascade called MAPK moves into the nucleus and activate TF by phosphorylation which bind to promoter elements to activate certain genes. For example NFkB remains inactive in cytosol by an inhibitor-I. Phosphorylation of the inhibitor frees the NFkB to move into the nucleus to activate few genes. In general signal cascade initiated by RTKs begins with small monomeric G-proteins called RAS.
RTK- Ras pathway:
Receptor Ras belongs to a super family of monomeric G-proteins related to another super family of heterotrimeric G-proteins, especially G-alpha. RAS members belong to a family of monomeric proteins such as Raf, Rab, CDC4 and Rho/Rac; all belong to the RAS super family. Rho and Rac relay signaling from surface receptors to actin cytoskeletons; and the members of Rab family of GTPases which are involved in trafficking intracellular proteins, but the Ras members which are located at the inner surface of plasma membranes initiate a kinase cascade which has tremendous effect on cellular metabolism as well as transcriptional activation of genes.
Ras gene was originally discovered as an oncogene causing cancer and found to be a normal cellular gene, but mutated. Ras is GTP binding monomeric protein (21kd) exists in Ras-GDP inactive and Ras-GTP active form. Ras itself as GTPase activity, converting GTP to GDP, it becomes inactive. Mutant Ras cannot hydrolyze GTP, thus remains active all the time. Ras members consists of 60 or so distinct members in mammalian systems, divided in to Ras, Rab, Rho, Arf, Ran and Gem subfamilies. In fact, plants like Arabidopsis contains 93 genes encoding GTP binding proteins (plants have all the said subfamily members but not Ras).
Activation of RTKs initiates with the binding of ligands and dimerization of RTK and autophosphorylation of its own cytosolic domain. Phosphorylation of receptor causes the binding of Grb2 protein which is also associated with Sos (son of seven). Thus Grbs-Sos binds to the p-lated RTK. The SOS then binds to inactive Ras which is anchored to the inner surface of the membrane. Binding of SOS to Ras leads to the release of GDP and the binding of GTP, thus Ras becomes active. The Ras now provides the binding sites for Raf, which is serine/threonine kinase; this initiates a chain of events while Ras is tyrosine kinase. Many growth hormones such as EGF binding to EGF receptor can lead to Ras independent pathway of activation. For example EGF, in C.elegans, controls contraction of smooth muscle cells. Such EGF, non RAS pathway, regulates the extrusion of oocytes from one compartment of bisexual gonads to the other compartment where they are fertilized. Coupling of EGF receptor to RAS is not actually required for EHF induced muscle contraction in many systems.
The RTK’s cytosolic phosphorylated tyrosine’s act as the binding site for GRB2 SH2 domain: GRB2 consists of two domains one called and SH2 and the other SH3. SH stands for Src homology. These protein domains are protein-protein interacting domains. The SH2 domain has a central antiparallel sheets flanked by 2 alpha helices. The target for this binding is a three a.a sequence pYEET where tyrosine phosphorylated. Similarly the GRB2 contains another domain called SH3, which is also protein- protein interacting domain, consists of 8-10 a.a sequence RXLPPLLXY or XXXPPLPXR. Thus GRB2 interacts with p-lated tyrosine in the cytosolic receptor domain and its end interacts with SOS which to has proline rich pocket for bind of GRB2. GRB2 acts as an adopter protein between RTK Receptor and SOS-Ras. Proteins do contain many such protein-protein interacting domains such as PH (pleckstrin homology), PID phospho tyrosine interacting domain (NPXPY).
The p-lated tyrosine binding to GRB2-SOS interacts with Ras-GDP, which is associated with inner surface of the membrane, thus induce RAS to exchange GDP with GTP (assisted by GTP exchange factor GEF), thus Ras become active.
A view of some of the proteins have specific Domain like SH2, SH3, PH, RG PTB and many such domains. Most of them have protein-protein interacting domains.
Ras is a monomeric G-protein a tyrosine kinase. Activated RAS-GTP transfers its Phosphorous to another accessory protein called Raf; this GTPase activity is assisted by GTPase called GAP. Ras contains about 170a.a and G-alpha contains 300a.a. yet their 3-D structure is similar and both belong to super family GTPase proteins.
Ras plays a crucial role in activating cell cycle events, if there is a mutation at 12th glycine it block the GTPase activity and RAS bound to GTP remains active for a long time; this can lead to cell proliferation and cancer. Mal function of RAS receptor can lead to cancer. Malfunction of GEF can lead to cancer. Malfunction of GAP can lead to cancer. Ras malfunction can lead to cancer, malfunctioning of it receptor leads to cancer, malfunctioning of its associated GEF and GAP leads to cancer. Mutation in Ras at 12 a.a glycine position renders Ras active all the time for the bound GTP remain bound for a long time; thus it is found to induce cancer; for that matter a majority of cancer types contain mutated Ras.
Raf leads to a sequence of events one leading to the other like a cascade. Raf is considered as MAP (mitogen activated protein kinase kinase kinase, MAPKKK); MAPKKK phosphorylates MAPKK which then phosphorylates MAP kinase (MAPK). Active Ras p-lates Raf which is serine/threonine kinase. Raf p-lates MEK (MEK is Tyrosine/serine/threonine kinase) and MEK-p plates MAPK (MAPK is serine/threonine kinase); this is a kinase cascade, ultimately the phosphorylation cascade ends in the nucleus; by the time the cascade reached the nucleus, it activates many other proteins laterally.
MAP kinase pathway: Ras-Raf mek signalling pathway (MAP Kinase pathway;
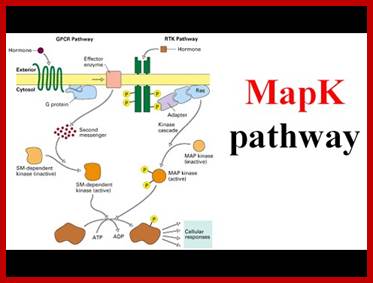
http://www.lady-ua.info; www.slideshare.com
In both cytokine receptor and RTK receptors signal transduction takes place via GRB2, SOS and Ras. Activated Ras promotes three sequentially acting protein kinase steps. The kinases are associated with a scaffold region. This cascade of activation of phosphorylation ends in MAP kinase, which is a serine/threonine kinase also known as ERK for this refers to external signal cascade. Activation of MAPK in different cell types can lead to similar or different responses.
Ras-Raf signal cascade:
Activated Ras which is bound by GTP binds to N-end of Raf (which is serine/threonine kinase). Hydrolysis of GTP releases Raf-GTP (active). Raf-GTP activates MEK, stands for MAPK, ERK kinase, (again by p-lation at serine threonine residues). MEK (a tyrosine kinase), then p-lates MAPK. MAPK (serine/threonine kinase) p-lates many other proteins including transcriptional factors. Mutated Raf with missing N-end can also induce cancer.
EGF, PDGF and few other growth hormones induce cells at Go to express more than 100 or more genes called early genes, to be induced before the cell enters into S-phase. One early response gene expressed is Fos gene. With other gene products such as Jun, Fos induces the expression genes required for progression through cell cycle. Majority of growth factors that stimulate transduction through RTKs induce cFos and few other similar genes. These genes have enhancer elements for growth factors like SRE (serum response elements). Many TF bind to such elements and some of them are activated by MAPK. It also activates a cytosolic kinase called c90 RSK, which translocates into the nucleus, where it p-lates specific serine residue in SRFs. MAPK a dimeric protein, also p-lates serine in TCF, which associates with two other p-lated SRFs, thus a trimeric complex binds to SRE elements of cFos gene. Some of the genes expressed are found to be TFs which in turn activate other required genes for cell proliferation.
For example one of the genes stimulated by MAPK is Fos, it is a proto-oncogene; can cause cancer. When the Fos protein combines with phosphorylated Jun, one of the nuclear proteins that is p-lated by MAPK, forms heterodimers and binds to gene regulatory element called AP1 and turns on the genes. The genes such as MYB and Myc are turned on by MAPK phosphorylation of TFs. In plants phytochrome, Gibberellins and other plant hormones work through such phosphorylation cascade and regulate gene expression by turning on MYB like TFs.
Though it is well recorded that RTKs generally produce a cascade effects ending is MAPK, several of trimeric G proteins too also induce signaling in MAPKs. E.g. Yeast mating pathway. Yeast and many other unicellular systems lack cytokine receptor or RTK pathways, yet they exhibit several alternative MAPK pathways.
GPCR and RTK Combined Activity:
Role and action of Yeast mating factors:
Even though each of the said GPCR and RTK receptor induced cascade is prevalent in many system one cannot exclude the cross activity of both, which both ultimately lead to nuclear transcriptional activities.
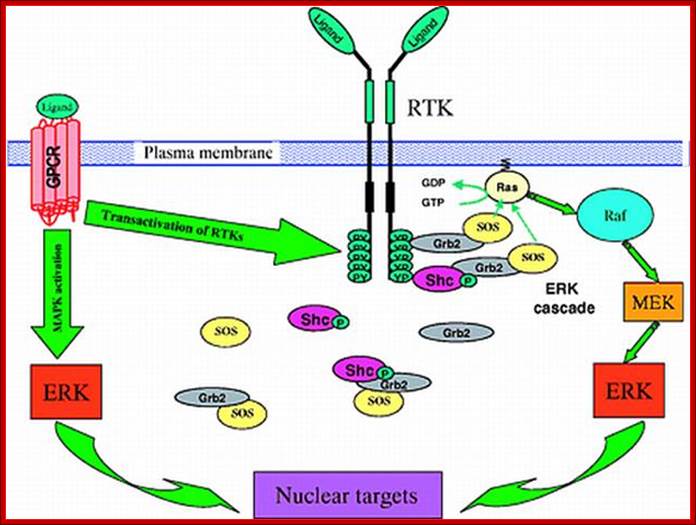
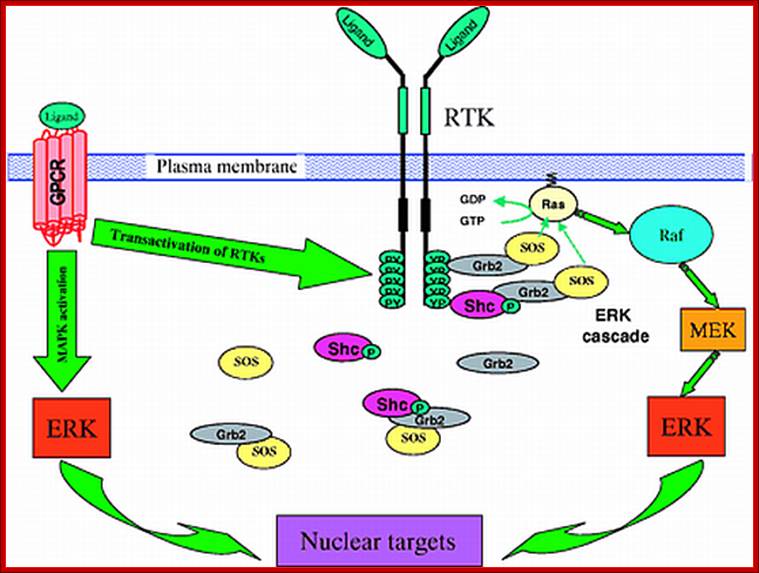
Signaling through the ERK (MAPK) cascade following activation of receptor tyrosine kinases (RTKs) and G-protein coupled receptors (GPCRs). RTKs activate the ERK cascade by recruiting guanine exchange factor SOS to the plasma membrane in close proximity to the membrane-anchored Ras. The upper right corner of the figure details the signaling activation route near the membrane surface. Below, it is shown that some of the signal transduction complexes, e.g. the Shc-Grb2-SOS and Grb2-SOS complexes, reside in the cytosol, raising the question of whether Ras activation occurs through direct interactions of SOS from the cytosol. Activated Ras-GTP promotes the activation of Raf-1, which phosphorylates MEK, which in turn activates ERK. Left, GPCRs stimulate MAPK cascades either through transactivation of RTKs, such as EGFR, or directly by using β-arrestin as a scaffold for the ERK and JNK cascades. For abbreviations, see list. See text for further details.
Four-dimensional organization of protein kinase signaling cascades: the roles of diffusion, endocytosis and molecular motors; ;http://jeb.biologists.org
Yeast mating factors is a paradigm for genetic transduction pathway in reproduction between two opposite strains. Mating factors secreted by yeast cells are ‘a’ and ‘alpha’. Haploid cells ‘a” secretes ‘a’ but contains ‘alpha ‘receptors. ‘Alpha cells secrete ‘alpha’ factors but they have ‘a’ receptors. Factor ‘a” bind to ‘alpha ‘receptors and ‘alpha’ factors bind to ‘alpha’ receptors. Activation of these receptor induce signal pathway leading to MAPK and lead to the expression of genes that inhibit progression into cell cycle and thus opposite mating types fuse to form 2n cells.

The Dbl family of guanine nucleotide exchange factors are multifunctional molecules that transduce diverse intracellular signals leading to the activation of Rho GTPases. The tandem Dbl-homology and pleckstrin-homology domains shared by all members of this family represent the structural module responsible for catalyzing the GDP–GTP exchange reaction of Rho proteins. Recent progress in genomic, genetic, structural and biochemical studies has implicated Dbl family members in diverse biological processes, including growth and development, skeletal muscle formation, neuronal axon guidance and tissue organization. The detailed pictures of their autoregulation, agonist-controlled activation and mechanism of interaction with Rho GTPase substrates, have begun to emerge. Y zheng; http://www.cell.com
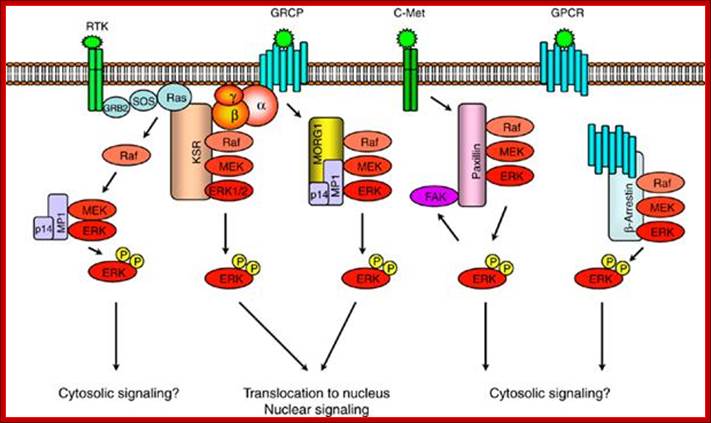
https://www.researchgate.net
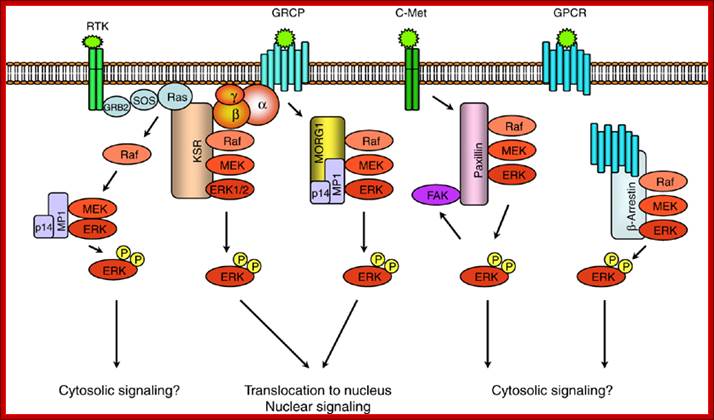
Scaffold proteins involved in ERK-signaling pathway. Scaffold protein KSR links signaling from RTKs and GPCRs to ERK-signaling modules. MORG1 specifically involved in linking GPCRs to ERK1/2 module. MP1 and p14 complex can interact with MORG 1 or RTK-signaling to direct the signal to MEK1-ERK1 signaling module. The scaffolding roles of paxillin and -arrestin appears to be involved in directing the signaling outputs from ERK-module to specific cytosolic compartments; https://www.researchgate.net
Ligand binding to these receptors, which are trimeric G proteins trigger GTP binding and activation where G-alpha-GTP dissociate from beta/gamma dimmers. This is similar to that of GPCR system, where induced G-alpha dissociates and activates signaling pathway. In yeast cells all physiological responses are mediated by yeast pheromones which act on G-bg. In yeast freed Gbg (beta and gamma subunits), triggers a kinase cascade analogous to Ras-Raf pathways. Analysis of ‘a’ and ‘alpha’ receptors and G-proteins, mutants show they contain ‘a’ and ‘alpha’ functional G-receptors, yet sterile and defective mating habits. In such cells G.bg are tethered to membranes via gamma subunit and activates Ste20 a protein kinase. It activates Ste 11; it is a serine/threonine kinase analogous to Raf or MEKK. Activated Ste11 plates and activates Fus3, which is ser/threonine kinase equivalent to MAPK. After translocation into the nucleus, Fus3 promotes expression of target genes by the p-lation of TFs such as Ste12. which is responsible for expression of mating factors? The other factors in yeast mating events are Ste5, which interacts with G-bg, Ste11, Ste7, Fus3, Ste5; they all act as scaffold for the assembly for other components becomes active G-alpha-GTP and G-bg dissociate but still held on to the membrane via gamma subunit. It provides a scaffold for the binding and activation of several factors such as Ste20 (ser/thr), Ste11 (mEKK, ser/thr), Ste7 (mek ser/thr), Fus3 (MAPK ser/thr), Ste12 (TF) in the given order or sequence. In these events the Sate5 provides scaffold for the assembly of all the above said complexes.
Note all MAPK kinase activation requires p-lation at Tyrosine/Thr at the lip region. MEK is a dual kinase (belong to another super family of proteins) plates several members of MAPK family. All signals end in one way or the other in MAPK as the end of the kinase cascade. Such system has been found in humans, drosophila and yeast. Specific kinases are activated in response to signals such as pheromones, starvation, high osmomolarity, hypotonic shock and carbon-nitrogen starvation. In both and higher EKs different MAPKs cascade share a cascade of common components.
|
Signal |
kinase |
response |
|
pheromones |
Fus3 |
mating |
|
starvation |
KSS1 |
filamentous |
|
High osmomolarity |
Hog1 |
Osmolyte synthesis |
|
Hypotonic shock |
MPK1 |
Cell wall dissolving |
|
Carbon. Nitrogen starvation |
Smk1 |
sporulation |
|
|
|
|
TGF beta Signal Transduction:
Transforming growth factor beta is an excellent example to illustrate its structure and action. TGFbeta belongs to a super family of ligands; one of them is bone morphogenic protein (BMP) which induces bone formation under in vitro cell culture. Presently BMP7 is used to strengthen the fractured bones. There are several BMP; some of them help in developing pathways such as development of mesoderm and blood forming cells. TGFbeta acts as a proliferative component in many mammalian cell types. TGF b also signals overcome TGFb induced growth inhibition. In Drosophila an equivalent of TGFb is DPP which controls dorso-ventral pattern formation. Though there a variety of TGFs, once they bind to the ligands downstream activity is more or less same.
Mechanism of action of TGF-beta in the promotion of hair growth, stimulating cell differentiation and extracellular matrix deposition;TGF bound to its receptors; http://www.doctorsequipe.it
Each of the TGFb is synthesized as precursor proteins with prodomain and processed into mature protein (mature domain). Secreted TGFb remains in ECM as inactive complex, for it is bound to latent TGFb binding protein (LTBP). A matrix protein like thromboprotein or integrin binding to the inactive TGFb induces a change in conformation as an active form. TGFb is 110-140a.a long has four antiparallel strands and 3 S-S bonds, such internal molecular bonds form what is called as cysteine knots, resistant to denaturation. An additional N-terminal S-S bond between two monomeric proteins makes it dimer. Binding of the ligand to its receptor has been demonstrated with FRET technology.
The receptor sits on the surface of the cell. The receptor proteins purified show proteins with different mol.wt 55, 85 and 280 Kda called R-1, R-2, and R-3. Among them R3 is the most abundant protein, it is called cell surface proteoglycan. It binds to TGFb and concentrates at cell surface. Type I and II are dimeric transmembrane receptor proteins with serine/threonine kinase activity at their cytosolic domain. R-II performs autocatalysis. Binding of the ligand induces formation of complexes containing each of the R1 and R-II, thus active RI kinase activity at the cytosolic domain. Phosphorylated domain interacts with cytosolic proteins such as Mads. There are three different kinds Mads proteins such as R-smad1, co sMads, and inhibitor sMads. Only sMads that is p-lated enters the nucleus along with smad-4. R-sMads consists of two domains called MH1 and MH2 separated by a flexible linker. The N-end of MH1 contain nuclear localization signals (NLS) and a DNA binding motif. The R-S mads contain two motifs one NLS and another DNA bonding motifs. When the smad is not p-lated its NLS is masked by the MH2 or called DNA binding motif. When R-sMads get p-lated at MH2 they open up and associate with importin-b, smad3 and smad 4 enter into the nucleus as a complex of 2smad-3 proteins and one smad-4 (co-smad) unit. Once they enter into the nucleus, they dissociate from b-importin and bind to DNA regulatory elements. They also get associated with other TFs and form a gene activation complex. Such activation complex, at plasminogen activator gene, activates an inhibitor gene called PA1-1. In the nucleus when the sMads are dephosphorylated they are exported back to the cytosol as dissociated subunits. So the concentration of active sMads depends on the level of active TGFb.
Cytokine signal transduction:
Interferons and cytokines are synthesized in response to infection at surfaces as the first line of defense what is called as innate immunity. Cytokines are a family of cellular secreted products that act as defense structure against viruses and bacteria within 12 to 24 hrs of infection. Cytokines induce signal transduction via specific cell surface receptors. The receptors bind to their respective cytokines and respond and transduce cellular responses. Receptors consist of extracellular domains, where they bind to ligands and a transmembrane domain and an extended cytoplasmic domain. A single ligand binding can induce dimerization and activation of kinase activity. Receptor proteins in a dimer can vary from one monomer to the other; for one of the monomer may contain JAK kinase activity at its cytosolic side and another receptor protein may possesses Tyrosine kinase activity. Both cytokine and RTK receptors once activated go through autophosphorylation (after dimerization) and act as binding or docking sites for proteins with SH2 domain such as IRS1 or multi docking protein via PTB domain.
Cytokines form a family of small secreted proteins of 160 a.a long. They control growth and differentiation of specific cell types. Prolactin, at the time of pregnancy, induces epithelial cells of immature ductless in the mammary gland into acinar cells that produces milk and secrete into the ducts. IL2 is required for the formation and function of T-cells. IL4 is required for growth and function of B-cells. Interferon alpha another form of cytokine, induced in response to viral infection of cells, once secreted acts on neighboring cells to become active and render them in anti viral state. Another cytokine called erythropoietin (epo) triggers the development of RBC from erythrocyte progenitor cell types in bone marrow. Erythropoietin is synthesized in kidney cells (which monitor the level of oxygen in the blood). Low oxygen induces oxygen sensitive TF called HIF1alpha to synthesize more erythropoietin and secrete into the blood. With this more progenitors are saved from death, allowing each cell to generate 50 or more RBC cells in a period 2 days. This way the body responds the loss of blood. Many cytokines induce differentiation of different types of blood cells, such as Granulocytes. Granulocyte colony stimulating factor (G-SF) induces specific progenitor in bone marrow to divide and differentiate into granulocyte s which is a kind of WBC that inactivate bacteria, virus and other pathogens. In cancer therapy specific GSFs are administered to stimulate proliferation and differentiation of granulocytes that leads to platelets formation required for clotting.

A glimpse at IFN pathways; http://www.onclive.com/
What Are Interferons?
IFNs were first characterized in 1957 and were named for
their role in a process called viral interference, in which one virus
interferes with the growth of another, unrelated virus. By the mid-1970s, IFN
research began to take off and, following reports of antitumor activity in
mice, they began to be used in the treatment of a wide range of different
cancers.
IFNs are part of the cytokine family of proteins⎯small,
hormone-like proteins that play an important role in communication between
cells and their external environment, produced in response to external stimuli.
They are classified into three groups according to which receptor they bind:
- Type I IFNs, which all use the type I IFN receptor, include IFNα, β, δ, ε, κ, and ω
- Type II IFNs, which use the type II IFN receptor, consist of IFNγ
- Type III IFNs, which use an IFNλ-specific receptor, consist of IFNλ
The
groups differ not just in which receptor they bind, but also in the cells that
produce them and in their genetic variation. While IFNγ is encoded by a
single gene, IFNλ has four subtypes, and IFNα has up to 14. The type
I IFNs are the most broadly expressed and can be produced by virtually any cell
in the body, though IFNα is produced primarily by macrophages and
dendritic cells, while IFNβ is expressed predominantly by fibroblasts. The
type II and type III IFNs are much more restricted in the types of cells that
both produce and respond to them.
IFNs exert their cellular effects by binding to
their specific receptor on the membrane of a target cell and initiating
intracellular signaling events. This is another important point of divergence
between the three groups. The type I and type III IFNs have significant overlap
in the downstream signaling events that they initiate. They activate the
tyrosine kinases Janus kinase 1 (JAK1) and tyrosine kinase 2 (TYK2), resulting
in the formation of heterodimers of signal transducer and activator of
transcription 1 and 2 (STAT1/2), which subsequently migrate into the nucleus
and associate with the transcription factor IFN regulatory factor 9 (IRF9) to
form the IFN-stimulated gene factor 3 (ISGF3) complex. This complex in turn
activates the transcription of IFN target genes. On the other hand, the type II
IFN, IFNγ, signals via JAK1 and JAK2 and activates different downstream
signaling events. - See more at: Jane de Lartigue, PhDhttp://www.onclive.com/
Almost all cytokine receptors contain similar tertiary structures in having 4 long helices folded into specific module. Each of them contains two extracellular sub domains containing beta strands folded together. In the case of erythropoietin receptor binding of the ligand to the receptor (single ligand binding to two receptor monomers) is typical of cytokine receptors (dimers).
Cells express specific receptor; the number and kind vary from one cell type to the other. Such cells respond to specific kind of cytokines depending upon the kind of TFs, chromatin nature and other proteins. Intracellular signaling pathways to different kinds of ligands and receptors are more or less similar. All eventually lead to the activation of TFs and gene expression.
For example Epo-EpoR and JAK2 pathway responds to Epo, binds to Epo-Receptor which activates JAK2 pathway. This pathway acts and uses STATs for transcriptional activation. This path way also activates RAS pathway through GRB2; with a cascade of kinase –kinase activity, activate MAPK kinase that activates several TFS; Activated JAK2 pathway and also activate Phospholipase-C. this can lead to rise in ca+ ion level which has tremendous effect on cellular activity including gene expression. The JAK2 also activates PI3 kinase which in turn activates a string of responses including activation of TFs.
JAK2 kinase and STAT pathway:
The cytokine receptors containing JAK2 activity have an exoplasmic ligand binding domain, a membrane pass domain and a cytosolic domain with kinase activity. The activated receptors having JAK kinase become dimmers and p-late their own Tyrosine residues. Tyrosine P-lated sites become the binding sites for SH2 domain containing STATs (stimulation of transcription and activation of transcription), which are a group of TFs. All STAT proteins contain an N-terminal SH2 domain, a central DNA binding domain and a tyrosine residue at C-end of the protein. Activated JAK2 p-lates tyrosine residue in STAT at C terminal. The p-lated STATs dissociate from the receptors and dimerize, where each p-lated SH2 domain binds p-lated site. Dimerization exposes NLS site for the import of the same into the nucleus. The STATs (they form different combination of STATS in different cell types) and bind to specific enhancer region and activate gene expression. Different combination of STATs expresses different genes. In erythroid progenitors STATs express BCLX gene, which prevent apoptosis and allow cell proliferation. Mice lacking STATs suffer from genetic anemia, yet mice survive but weak.
The downstream activation of Epo-EpoR-Jak2 pathway is multifold. The cytosolic p-lated domain binds with an adapter protein GRB2, which reacts with Ras. Ras activates MAP kinase that leads to activation of Transcription. This also activates Phospholipase-C which results in opening of Ca= channels and calcium level increase. This can lead to regulation of gene expression. JAK2 also activates PI3 kinase, which activates proteinase B which can lead to activation or repression of few cellular proteins.
Cytokine signals are modulated in the sense STATs activated transcriptional activity is time bound, not too long nor too short. This is regulated by SHp1 phosphotase. In mutant mice, for SHp1 dies, because of excess production of erythrocytes and other cells. SHp1 negatively regulates several kinds of cytokine receptors in progenitor cells. In the case of Epo stimulated cells, receptor bound JAK kinase2 is dephosphorylated by SHp1, which has p-tyrosine binding domain at one side and at the end it contains phosphotase domain. Use of these domains the JAK kinase is regulated.