Immunology 6
Immune Diseases and Cure:
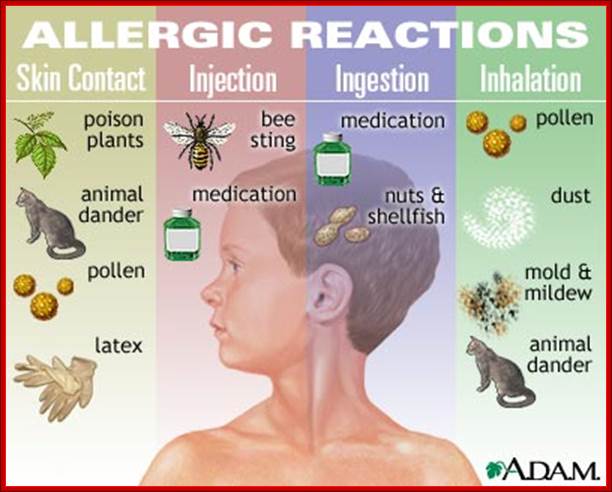
Hypersensitivity:
Immediate hypersensitivity is an immune reaction triggered by antigen binding to IgE pre attached to mast cells that lead to inflammatory mediator release.
The steps in the development immunity are exposure to an antigen (allergen) that stimulate Th2 cell responses and IgE production, binding of IgE to FcE receptors on mast cells, cross linking of IgE and FcE receptors by the allergens, activation of mast cells and release of mediators.
Individuals susceptible to immediate hypersensitivity reactions are called atopic and often have more IgE specific Fc receptors per mast cell than nonatopic individuals. IgE synthesis is induced by exposure to antigens and Th2 cytokines particularly IL-4.
Many cells derived from the bone marrow and mature in the tissues. They express high affinity receptors for IgE (FcERI) and contain cytoplasmic granules in which are stored various inflammatory mediators. Subsets of mast cells including mucosal and connective tissues mast cell, may produce different mediators. Basophils are a type of circulating granulocytes that express high affinity FcE receptors and contain granules with content similar to mast cells.
Eosinophils are a special class of granulocytes, for they are recruited into inflammatory reactions by chemokines and IL-4 and are activated by IL5. Eosinophils are effector cells of IgE –initiated reactions. They mediate IgE directed ADCC to eradicate parasites. In allergic reactions Eosinophils contribute to tissue injury.
On binding of antigens to IgE on the surface of Basophils, the high affinity FcE receptors become cross linked and activate intracellular second messengers that lead to granule release and fresh synthesis of mediators. Activated mast cells and Basophils produce three important classes of mediators; biogenic amines, such as histamine, lipid mediators such as prostaglandins, leukotrienes and PAF; and cytokines mediate the late phase reactions.
Various organs show distinct forms of immediate hypersensitivity involving different mediators and target cell types. Any allergen may lead to a systemic reaction called anaphylactic shock. Asthma is a manifestation of immediate hypersensitivity and late phase reactions in the lung... Allergic Rhinitis (hay fever) is the most common allergic disease of the upper respiratory tract. Food allergens can cause diarrhea and vomiting. In the skin immediate hypersensitivity is manifested as wheal flare and late phase reactions and may lead to chronic eczema.
Drug therapy is aimed at inhibiting mast cell mediator production and at blocking or counteracting the effects of released mediator on target cells/ organs. The goal of immunological therapy is to prevent or to reduce Th2 cell responses to specific allergens and the production of IgE.
Immediate hypersensitivity reactions provide protection against helminths by promoting IgE and eosinophil-mediated ADCC and gut peristalsis. Furthermore, mast cells may play an important role in innate immune responses to bacterial infections.
Transplantation of tissues from one individual to a genetically non identical recipient leads to a specific immune response called graft rejection that can destroy the graft. The major molecules target in transplantation/rejection is allogenic class I and II MHCs.
Many different T cell clones present, which are specific for different foreign antigens plus self MHC s ell, including CTLs that kill graft cells and helper T cells that causes many cross reactions with an individual allogenic MHC molecules. This high frequency of T cells capable of directly recognizing allogenic MHC s explains why the response to allo-antigens is much stronger than response to normal antigens.
Allogenic MHC molecules may be found/ presented on donor APCs to recipient T cells (the direct pathway), or the allo-antigens may pick up by host APCs that enter the graft or reside in draining lymphoid organs and be processed and presented to T cells as peptides associated with self MHCs ( indirect pathway).
Graft rejection is mediated by Tells including CTLs that kill graft cells and helper cells that cause DTH reactions and by Abs.
Several effector mechanisms cause rejection of solid organ grafts and each mechanism may lead to a histologically characteristic reactions. Preexisting Abs cause rejection characterized by thrombosis of graft vessels. Allo reactive T cells and antibodies produced in response to graft cause blood vessel walls damage and parechymal cell death called acute rejection. Chronic rejection is characterized by fibrosis and vascular abnormalities (accelerated arteriosclerosis), which may prevent a chronic DTH reaction in walls of arteries.
Rejection may be prevented or treated by immunogenicity of graft (e.g. by limiting MHC allelic differences), or by induction of tolerance. Most of the immunosuppressant is directed at T cell responses and entails the use of cytotoxic drugs, specific immunosuppressive agents or anti T cell Abs. The most widely used immunosuppressive agent is cyclosporine, which blocks T cell cytokine synthesis. Immunosppression is often combined with anti-inflammatory drugs such as corticosteroids that inhibit cytokine synthesis.
Patients receiving solid organ transplants may become immunodificient because of their therapy and are susceptible to viral infections and virus related malignant organs as well as the lungs and may also be fatal. Xenogenic transplantations of solid organs is limited by the presence of natural Abs to carbohydrate antigens on the cells of discordant species that cause hyper acute rejection, antibody-mediated acute vascular rejection and strong T cell mediated immune response to xenogenic MHC molecules.
Bone marrow transplantations are susceptible for rejection and the recipients require intense preparatory immunosuppression. In addition T/Ls in B/m graft may respond to allo-antigens of the host and produce GVHD. Acute GVHD is characterized by epithelial cell death in the skin, intestinal tract and liver; it may also be fatal. Chronic GVHD is characteristic by fibrosis and atrophy of one or more of these same target organs as well as the lungs and may also be fatal. Bone marrow transplant recipients also often develop severe immunodeficiency rendering them susceptible to infections.
Immunity and Tumors:
Tumors express antigens that are recognized by immune system but most tumors is weekly antigenic and immune response often fail to prevent the growth of tumors. Immune system can be stimulated to effectively kill tumors.
Tumor antigens are recognized by CTLs, they are principal inducers of and targets for autoimmunity. These antigens include mutants of oncogenes and other cellular proteins, a normal protein whose expression is dysregulated or increased in tumors and products of oncogenic viruses.
Antibodies specific for tumor cells recognize antigens that are used for diagnosis and potential targets for antibody therapy. These Abs include oncofetal antigens, which are expressed normally during the fetal life and whose expression is deregulated in some tumors, altered surface glycoproteins and glycolipids and molecules that are normally expressed on cells from which the tumors arise are thus differentiation antigens to particular cell types.
Immune responses that are capable of killing tumor cells consists of CTLs, NK cells activated macrophages (MPs). The role of these immune effector mechanism protecting individuals from tumors is not well understood nor understood.
Immuno-therapy for tumors is designed to augments active immune responses against these tumors or to administer tumor specific immune effectors to patients. Immune responses may be actively enhanced by vaccination with tumor cells or antigens, administration of tumors modified to express high levels of co stimulators or cytokines that stimulate T cell proliferation and differentiation and systemic administration of cytokines. Approaches for passive immunotherapy includes the administrations of antitumor antibodies , and antibodies conjugated with toxic drugs (immunotoxins) and tumor reactive T cells and NK cells isolated from patients and expand by culture with growth factors.
Immune diseases: Disorders by abnormal immune responses are called hypersensitivity diseases. Pathologic immune responses may be autoimmune responses directed against self antigens or uncontrolled and excessive responses to foreign antigens.
Hypersensitivity diseases may result from Abs that bind to cells or tissues circulating immune complexes that are deposited in tissues to T/cell reactive with antigens.
The effector mechanisms of AB mediated tissue injury are complement activation and Fc receptor-mediated inflammation. Some Abs cause disease by interfering with normal cellular functions without producing tissue injury. The effector mechanism of T/c-mediated tissue injury is DTH reactions and cell lyses by CTLs.
Autoimmunity results from a failure of self tolerance. Autoimmune reactions may be triggered by environmental stimuli, such as infections in genetically susceptible individuals.
Most autoimmune disease is pathogenic and numerous susceptibility genes contribute to disease development. The greatest contribution is from MHC genes; other genes are believed to influence the selection of self-reactive lymphocytes and development of self tolerance.
Infections may predispose to autoimmunity by several mechanisms including enhanced expression of co stimulators in tissue and cross-reactions between microbial antigens and self antigens.
The current treatment for autoimmune disease is targeted at minimizing the injurious consequences of the autoimmune reactions. The future goal of therapy is to inhibit the responses of lymphocytes specific for self antigens and to induce tolerance in these cells.
Acquired and congenital immunodifficiencies:
Immunodeficiency (ID) disease are the caused by congenital or acquired defects in L/c., phagocytes and other mediators of adaptive and innate immunity. These diseases are associated with an increased susceptibility to infections, the nature and severity of which depend largely on which component of the immune system is abnormal and extent of the abnormality.
Defects in L/C development can affect both T and B cells (X linked and autosomal form of SCID), only B cells (X linked agammaglobulinemia) or only T cells (DiGeorge syndrome).
Defects in lymphocyte activation can affect L/C responses to antigens, and antigen presentation (e.g., bare lymphocyte syndrome of TAP deficiency) or T cell-B cell collaboration (X-linked hyper IgM syndrome).
Defects in phagocytes include defects in microbial killing (e.g., CGD or Chediak-Higashi syndrome) and defects in leukocyte migration and adhesion (e.g., LAD).
Treatment of congenital immunodifficiencies involves transfusion of Igs, Bone marrow stem cell transplantation or enzyme replacement. Gene therapy may offer improved treatment in future.
Acquired ImD are caused by infections, malnutrition, disseminated cancer and immunosuppressive therapy for transplant rejection or autoimmune deficiency disease.
AIDS is a sever ImD caused by the infection of HIV. This RNA virus infects CD4+T lymphocytes, macrophages and dendrite cells and causes massive dysfunction of adaptive immune system. Most of ImD in AIDS can be ascribed to the depletion of CD4+T cells.
HIVs enter the cells by binding to both CD4 molecules and co receptor of the chemokine family. Once inside the cell the viral genome is reverse transcribed into DNA and incorporated into the cellular genome by random insertions. Viral reproduction is stimulated by signals that normally activate host cells. The viral gene transcription and production of viruses is accompanied with cell death.
CD4 T+ cells depletion in HIV infected individuals is due to direct cytopathic effects of the virus, toxic effects of viral products such as shed gp120 and indirect effects such as activation-induced cell death or CTL killing of infected CD4+ cells.
Several reservoirs of HIV exist in the body. Including short lived but activated CD4-T cells, long live macrophages and very long lived latently infected resting T cells.
HIV induced depletion of CD4T cells result in greatly increased susceptibility to infections by a number of opportunistic microorganisms. In addition, HIV infected patients have an increased incidence of tumors particularly Kaposi’s sarcoma and EBV associated B cell lymphomas and encephalopathy; the mechanisms by which these are affected is not known.
HIV has a high mutation rate, which allows the virus to evade host immune system responses as well as drug therapies. Genetic variability also poses a problem for the design of an effective vaccine against HIV. The viral infection can be treated by the combination of inhibition of viral enzymes (how?).
The best treatment for this disease is a bone marrow transplant (BMT) , which, in most cases, would cure the problem. A bone marrow transplant involves taking cells that are normally found in the bone marrow (the soft, spongy tissue found inside the bones that is responsible for the development and storage of blood cells), and giving them back either to the patient or to another person. The goal of bone marrow transplantation is to infuse healthy bone marrow cells into a person after their own unhealthy bone marrow has been eliminated. BMT would help increase the sick child's immune system.
http://www.nlm.nih.gov/
Bone Marrow Cells; http://www.res.rcs.k12.tn.us/
http://medical-dictionary.thefreedictionary.com/bone+marrow
http://kevorganism.blogspot.com/
How is SCID treated?

Bobble boy disease; Severe combined immune deficiency; http://www.health-writings.com/
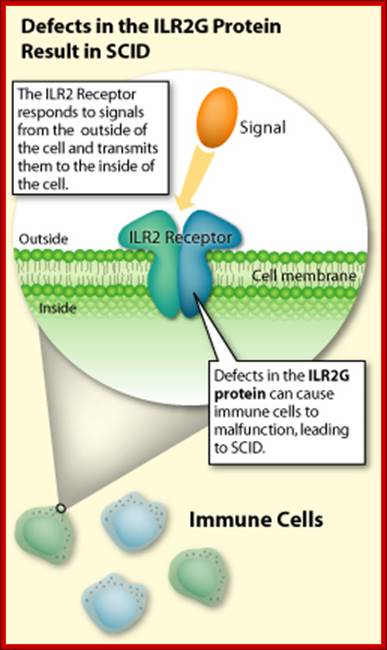
http://learn.genetics.utah.edu/
Children with SCID must be careful to stay away from germ-rich environments (such as day care centers and crowded shopping malls) where they could pick up a potentially life-threatening infection.
The most effective treatment is a bone marrow transplant. Unspecialized stem cells (that will form blood and immune cells) are taken from the bone marrow of a healthy donor and injected into the SCID patient. Ideally, these new cells will stimulate the production of the needed immune cells. Transplants done within the first few months of life are most successful. The tissue must be "matched" to the patient, however, which can limit the usefulness of this therapy. Siblings make the best donors as their cells likely contain a similar genetic makeup.
Gene therapy for this disorder may soon be possible. This therapy would compensate for the faulty gene by injecting healthy copies of the gene into a patient's bone marrow stem cells.
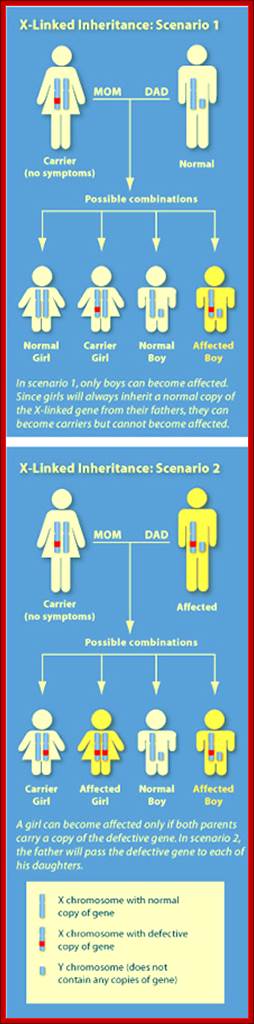
http://learn.genetics.utah.edu/

http://www.nature.com/
In
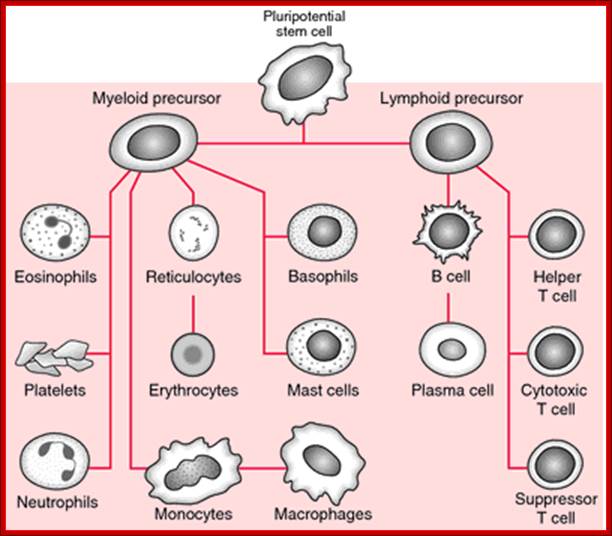
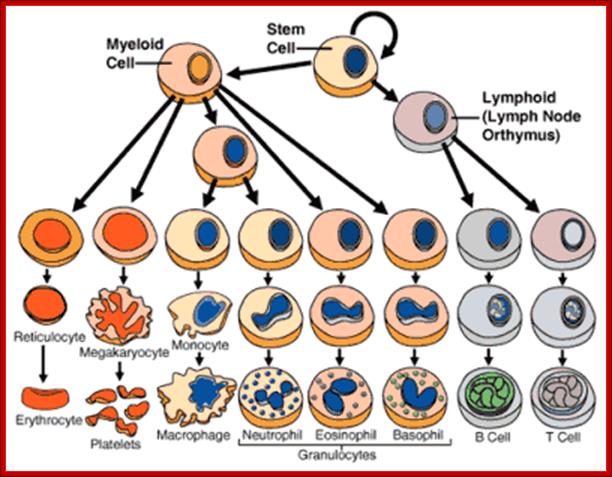
patients with severe combined immune deficiency (SCID), blocked lymphocyte
production leads to immune deficiency. Blood cells are produced by the
proliferation and differentiation of pluripotent haematopoietic stem cells
(HSCs) through stages of lineage-restricted progenitors, the common lymphoid
progenitor (CLP) and the common myeloid progenitor (CMP).These yield mature
blood cells, including B cells (B), T cells (T), natural-killer cells (NK),
granulocytes (Gran), monocytes (Mono), platelets (Plat) and erythrocytes
(RBCs). Inherited mutations of genes that are needed for the production,
survival or function of lymphocytes can cause severe combined immune deficiency
(SCID), with absent or non-functional B, T and NK cells. b | The block
to immune-cell production that occurs in patients with SCID leads to expansion
of the progenitor-cell pool (CLPs). The number of CD34+
haematopoietic stem and progenitor cells obtained from the bone marrow of the
youngest infant with X-linked SCID was relatively high for their size. This
finding indicates that the pool of progenitor cells could be expanded in
patients with X-linked SCID because of the absence of the ![]() c-chain, which is required for cytokine signalling and
differentiation of CLP. c | Gene correction of haematopoietic and
progenitor cells leads to immune reconstitution. In gene therapy for X-linked
SCID, a retroviral vector was used to transfer the normal human
c-chain, which is required for cytokine signalling and
differentiation of CLP. c | Gene correction of haematopoietic and
progenitor cells leads to immune reconstitution. In gene therapy for X-linked
SCID, a retroviral vector was used to transfer the normal human ![]() c cDNA into HSCs that were isolated from the patient's bone
marrow. Expression of
c cDNA into HSCs that were isolated from the patient's bone
marrow. Expression of ![]() c restores the cytokine response and allows differentiation of
CLP to B, T and NK cells, yielding immune reconstitution. The success in immune
reconstitution in these patients might be due to the large pool of progenitor
cells that were available for transduction, and to the high level of
engraftment of the genetically altered cells, due to the absence of endogenous
mature lymphocytes.
c restores the cytokine response and allows differentiation of
CLP to B, T and NK cells, yielding immune reconstitution. The success in immune
reconstitution in these patients might be due to the large pool of progenitor
cells that were available for transduction, and to the high level of
engraftment of the genetically altered cells, due to the absence of endogenous
mature lymphocytes.
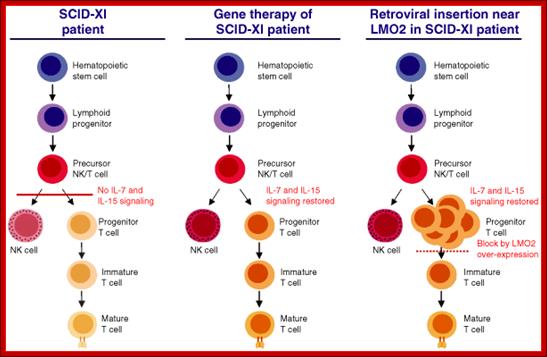
Figure 3.
Model for leukemogenesis in X-linked severe combined immunodeficiency (SCID-XI) gene therapy:
Left panel: T and natural killer (NK) cells are lacking when IL2RG is absent or not working properly. Middle panel: T and NK cell development is restored after retroviral SCID-XI gene therapy. Right panel: Overexpression of LMO2 causes a partial block in early T cell development. IL=Interleukin.
https://lookfordiagnosis.com

http://en.wikibooks.org/wiki/Structural_Biochemistry/DNA_recombinant_techniques.

http://en.wikibooks.org/wiki/ Structural_Biochemistry/DNA_recombinant_techniques
Use the same construct and introduce the recombined gene into islets of Langerhans in site specific manner and put them back into patients.

http://mynotes4usmle.tumblr.com/
Radial deviation of WRIST Joint deformities
· Radial deviation of WRIST; Ulnar deviation of FINGERS
· Boutonnière deformity: PIP flexion + DIP hyperextension
· Swan neck deformity: PIP hyperextension + DIP flexion
"This
study helps extend gene treatment research to nongenetic, nonlethal
diseases," explains principal investigator Christopher Evans, PhD,
Director of the Center for Advanced Orthopedic Studies at BIDMC.
"Rheumatoid arthritis [RA] is an extremely painful condition affecting
multiple joints throughout the body. Arthritis is a good target for this
therapy because the joint is a closed space into which we can inject
genes," adds Evans, who is also the Maurice Muller Professor of Orthopedic
Surgery at Harvard Medical School.
A classic autoimmune disease, RA develops when, for unknown reasons, the body's
immune system turns against itself, causing joints to become swollen and
inflamed. If the disease is inadequately controlled, the tissues of the joint
are eventually destroyed. Even though anti-inflammatory agents and biologics
can help to mitigate symptoms, there is no cure for the condition, estimated to
affect more than 2 million individuals in the U.S. alone.
Evans has spent a number of years studying the molecules responsible for the
breakdown of cartilage in patients with arthritis, identifying interleukin-1 as
a good target. But, he adds, once he had this answer, another question was not
far behind: How could he effectively reach the joints to block the actions of
this protein?
Gene treatment provided the answer. By implanting a gene in the affected joint,
he was able to stimulate production of a human interleukin-1 receptor
antagonist protein, which serves to block actions
of the interleukin-1 protein.
"The idea is that by remaining in place, the new gene can continuously
block the action of the interleukin-1 within the joints," says Evans.
"In essence, the gene becomes its own little factory, continuously working
To alleviate pain and swelling”
In
2005, as per a research findings reported in the Proceedings of the National
Academy of Sciences (PNAS), Evans and his colleagues demonstrated
that the IL-1Ra gene could be safely transferred to human joints in patients
with RA. In this new paper, the authors aimed to prove that the treatment was
not only safe, but that it was of therapeutic benefit. Two study subjects were
recruited. (The number reduced from six study subjects following severe adverse
events in an unrelated gene treatment trial taking place elsewhere, as per
Evans.) Both subjects were postmenopausal females under the age of 75 with a
diagnosis of advanced rheumatoid arthritis. After tissue was removed from the
subjects' knuckle joints, a harmless retrovirus was inserted into the tissue
cells, in order to serve as a "vector" to transport the gene into the
patients' joints. After being placed in culture to grow and replicate, the
cells were injected back into the afflicted joints.
After four weeks, patients reported reduced pain and swelling, as per Evans.
"In one of the two subjects, these effects were dramatic, and the
gene-treated joints remained pain-free even though other joints experience
flares." Subsequent laboratory tests showed that tissues removed from the
subject's joint tissue synthesized lower amounts of disease-related proteins,
confirming that the reduction in pain and swelling resulted from the actions of
the implanted gene.
"Existing therapies for rheumatoid arthritis are costly and need to be
administered regularly," says Evans, adding that in addition to risk of
side effects, not all patients respond well. This paper provides us with the
first real evidence that painful symptoms can indeed be lessened through gene treatment.
Ongoing work will focus on the use of gene treatment for the therapy of
osteoarthritis, as well as rheumatoid arthritis.
Engineering Immunity:
The immune system is a sophisticated machine, designed to fend off a constant barrage of disease-causing microbes. Unfortunately, it's not as good at fighting cancer, which disguises itself as normal tissue. Using gene therapy, Lilli Yang is reprogramming the immune system to recognize and kill cancer cells.
Stimulating the immune system to fight cancer is one of today's hottest research areas. Some scientists hope to genetically modify patients' white blood cells to do the job, but Yang is altering the body's blood-forming stem cells, a technique that could prove much more powerful. Because stem cells are self-renewing, they could generate a lifelong supply of immune cells programmed to combat, or even prevent, the disease.
A project manager and lead scientist in Caltech's Engineering Immunity Program, Yang created a viral "vector" that simultaneously delivers two genes to the stem cells: the genes encode the T-cell receptor protein, which enables white blood cells called T cells to recognize and kill cancer cells. The modified stem cells then give rise to T cells that bear the receptor. The technique has so successfully suppressed tumors in mice that Yang plans to begin trials this spring in melanoma patients.
In order to treat patients, Yang will have to isolate and modify their blood-forming stem cells in the lab and then reinjection them--a laborious, costly process. So she is collaborating with her husband, Pin Wang of the University of Southern California, to design viral vectors that can deliver therapeutic genes to only one cell type. They have succeeded in mice, a significant advance in gene therapy.
In the future, says Yang, treating cancer could be as simple as injecting patients with such targeted vectors. Gene therapy has yet to live up to its huge potential, but "Lilli has the ability to make it a part of modern medicine," says Caltech biologist and Nobel Prize winner David Baltimore, Yang's supervisor. Indeed, Yang is also creating vectors to stimulate specific immune cells to make antibodies against HIV. If successful, that project could at last lead to an AIDS vaccine.

Credit: Lili Yang, Steven Lee, and Sungjin Park; http://www2.technologyreview.com
Engineering immunity: Caltech's Engineering Immunity project, which Lilli Yang heads, aims to combine immunotherapy, gene therapy, and stem cell therapy to treat diseases such as HIV and cancer. The lower-right image shows that, first, cancer-specific T cell receptor genes or HIV-specific antibody genes are extracted from disease-specific white blood cells. At left, these genes are delivered to the blood-forming stem cells found in bone marrow. In the top image, the genes program the stem cells to develop into disease-battling white blood cells called T lymphocytes (for cancer) or B lymphocytes (for HIV). Since the blood-forming stem cells can survive throughout the lifetime of a person, such gene therapy could potentially provide lifelong protection against cancer or HIV.

Viruses are ideal vectors for gene therapy. By Tom Scott; http://ghr.nlm.nih.gov/
What is Gene Therapy?
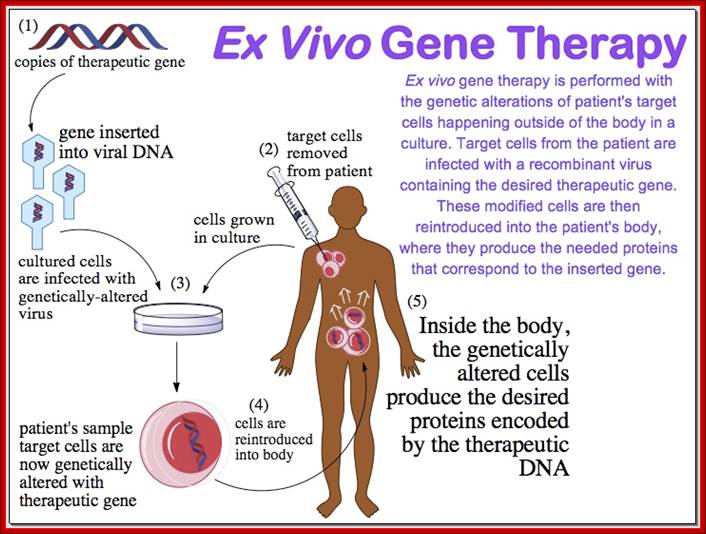
Gene therapy is the experimental treatment process of inserting a “corrected” or therapeutic gene into an individual’s cells and tissues to replace an “abnormal” disease-causing gene in order to treat a disease, or to use a gene to treat a disease, just like a medicine. Although gene therapy is still in its infancy, it has shown great potential in correcting and replacing defective genes behind many diseases, including cancer.
One way gene therapy is accomplished is through the use of a carrier or “biological vector” that delivers the therapeutic gene to the patient’s target cells. Commonly used carriers are viruses that have been genetically altered to carry normal human DNA. Since viruses are capable of encapsulating and delivering their genes by tricking cells into accepting them as part of their own genetic material, doctors and molecular biologists are using this power to their advantage by manipulating and re-engineering viruses and replacing their disease-causing genes with therapeutic ones. During this process, target cells are infected with the re-engineered viral carrier, which then unloads its genetic material into the cells’ nuclei. This is a complex procedure that must be performed in a precise manner so that the viral genome (hereditary information or complete DNA of one set of chromosomes) can be delivered and the therapeutic proteins expressed in the host cells. This has been achieved for many viruses that can now be used as vectors, including the virus causing HIV, and adenovirus.
![[Dr. Gero Hutter]](Cell_And_Molecular_Immunology6-Immune_Diseases_And_Cure_files/image017.jpg)
Sixten Koerper
Dr. Gero Hütter isn't an AIDS specialist, but he 'functionally cured' a patient, who shows no sign of the disease.
"I was very surprised," said the doctor, Gero Hütter.
The breakthrough appears to be that Dr. Hütter, a soft-spoken hematologist who isn't an AIDS specialist, deliberately replaced the patient's bone marrow cells with those from a donor who has a naturally occurring genetic mutation that renders his cells immune to almost all strains of HIV, the virus that causes AIDS.
The development suggests a potential new therapeutic avenue and comes as the search for a cure has adopted new urgency. Many fear that current AIDS drugs aren't sustainable. Known as antiretroviral, the medications prevent the virus from replicating but must be taken every day for life and are expensive for poor countries where the disease runs rampant. Last year, AIDS killed two million people; 2.7 million more contracted the virus, so treatment costs will keep ballooning.
While cautioning that the Berlin case could be a fluke, David Baltimore, who won a Nobel Prize for his research on tumor viruses, deemed it "a very good sign" and a virtual "proof of principle" for gene-therapy approaches. Dr. Baltimore and his colleague, University of California at Los Angeles researcher Irvin Chen, have developed a gene therapy strategy against HIV that works in a similar way to the Berlin case. Drs. Baltimore and Chen have formed a private company to develop the therapy.
Back in 1996, when "cocktails" of antiretroviral drugs were proved effective, some researchers proposed that all cells harboring HIV might eventually die off, leading to eradication of HIV from the body -- in short, a cure. Those hopes foundered on the discovery that HIV, which integrates itself into a patient's own DNA, hides in so-called "sanctuary cells," where it lies dormant yet remains capable of reigniting an infection.
But that same year, researchers discovered that some gay men astonishingly remained uninfected despite engaging in very risky sex with as many as hundreds of partners. These men had inherited a mutation from both their parents that made them virtually immune to HIV.
The mutation prevents a molecule called CCR5 from appearing on the surface of cells. CCR5 acts as a kind of door for the virus. Since most HIV strains must bind to CCR5 to enter cells, the mutation bars the virus from entering. A new AIDS drug, Selzentry, made by Pfizer Inc., doesn't attack HIV itself but works by blocking CCR5.
About 1% of Europeans, and even more in northern Europe, inherit the CCR5 mutation from both parents. People of African, Asian and South American descent almost never carry it.
Dr. Hütter, 39, remembered this research when his American leukemia patient failed first-line chemotherapy in 2006. He was treating the patient at Berlin's Charité Medical University, the same institution where German physician Robert Koch performed some of his groundbreaking research on infectious diseases in the 19th century. Dr. Hütter scoured research on CCR5 and consulted with his superiors.
Finally, he recommended standard second-line treatment: a bone marrow transplant -- but from a donor who had inherited the CCR5 mutation from both parents. Bone marrow is where immune-system cells are generated, so transplanting mutant bone-marrow cells would render the patient immune to HIV into perpetuity, at least in theory.
There were a total of 80 compatible blood donors living in Germany. Luckily, on the 61st sample he tested, Dr. Hütter's colleague Daniel Nowak found one with the mutation from both parents.
To prepare for the transplant, Dr. Hütter first administered a standard regimen of powerful drugs and radiation to kill the patient's own bone marrow cells and many immune-system cells. This procedure, lethal to many cells that harbor HIV, may have helped the treatment succeed.
The transplant specialists ordered the patient to stop taking his AIDS drugs when they transfused the donor cells, because they feared the powerful drugs might undermine the cells' ability to survive in their new host. They planned to resume the drugs once HIV re-emerged in the blood.
But it never did. Nearly two years later, standard tests haven't detected virus in his blood, or in the brain and rectal tissues where it often hides.
The case was presented to scientists earlier this year at the Conference on Retroviruses and Opportunistic Infections. In September, the nonprofit Foundation for AIDS Research, or am FAR, convened a small scientific meeting on the case. Most researchers there believed some HIV still lurks in the patient but that it can't ignite a raging infection, most likely because its target cells are invulnerable mutants. The scientists agreed that the patient is "functionally cured."
Caveats are legion. If enough time passes, the extraordinarily protean HIV might evolve to overcome the mutant cells' invulnerability. Blocking CCR5 might have side effects: A study suggests that people with the mutation are more likely to die from West Nile virus. Most worrisome: The transplant treatment itself, given only to late-stage cancer patients, kills up to 30% of patients. While scientists are drawing up research protocols to try this approach on other leukemia and lymphoma patients, they know it will never be widely used to treat AIDS because of the mortality risk.
There is a potentially safer alternative: Re-engineering a patient's own cells through gene therapy. Due to some disastrous failures, gene therapy now "has a bad name," says Dr. Baltimore. In 1999, an 18-year-old patient died in a gene therapy trial. Even one of gene therapy's greatest successes -- curing children of the inherited "bubble boy" disease -- came at the high price of causing some patients to develop leukemia.
![[Chart]](Cell_And_Molecular_Immunology6-Immune_Diseases_And_Cure_files/image018.jpg)
http://www.medibolics.com
Gene therapy also faces daunting technical challenges. For example, the therapeutic genes are carried to cells by re-engineered viruses, and they must be made perfectly safe. Also, most gene therapy currently works by removing cells, genetically modifying them out of the body, then transfusing them back in -- a complicated procedure that would prove too expensive for the developing world. Dr. Baltimore and others are working on therapeutic viruses they could inject into a patient as easily as a flu vaccine. But, he says, "We’re a long way from that."
Expecting that gene therapy will eventually play a major role in medicine, several research groups are testing different approaches for AIDS. At City of Hope cancer center in Duarte, Calif., John Rossi and colleagues actually use HIV itself, genetically engineered to be harmless, to deliver to patients' white blood cells three genes: one that inactivates CCR5 and two others that disable HIV. He has already completed the procedure on four patients and may perform it on another.
One big hurdle: doctors can't yet genetically modify all target cells. In theory, HIV would kill off the susceptible ones and, a victim of its own grim success, be left only with the genetically engineered cells that it can't infect. But so far that's just theory. All Dr. Rossi's patients remain on standard AIDS drugs, so it isn't yet known what would happen if they stopped taking them.
In 1989, Dr. Rossi had a case eerily similar to the one in Berlin. A 41-year-old patient with AIDS and lymphoma underwent radiation and drug therapy to ablate his bone marrow and received new cells from a donor. It is not known if those cells had the protective CCR5 mutation, because its relation to HIV hadn't been discovered yet. But after the transplant, HIV disappeared from the patient's blood. The patient died of his cancer 47 days after the procedure. Autopsy tests from eight organs and the tumor revealed no HIV
http://www.pennmedicine.org/
New lipid molecule holds promise for gene therapy:

Nanoscale assembly of the new lipid (green) and DNA (purple). Credit: Peter Allen, UCSB. Copyright 2006 American Chemical Society March 22nd, 2006
Read more at: https://phys.org/news/2006-03-lipid-molecule-gene-therapy.html#jCp
Nanoscale assembly of the new lipid (green) and DNA (purple). Credit: Peter Allen, UCSB. Copyright 2006 American Chemical Society
Scientists at the University of California, Santa Barbara have created a new molecule that holds promise in fighting disease via gene therapy. Inherited diseases, as well as many cancers and cardiovascular diseases, may eventually be helped by this approach, which delivers therapeutic genes directly to cells. These genes can correct genetic defects, for example, or help the body's immune system fight cancer cells.
Gene Analysis Software: The Leading Tool for Academic Labs. Download Your Free Trial Today! - www.ingenuity.com
Great Peptides - Beta Amyloids In Stock Free Shipping in Large Orders - www.CaliforniaPeptide.com
For more than two decades, gene delivery has been accomplished by using engineered viruses as a vehicle to get into diseased cells and 70 percent of clinical trials worldwide continue to use this method. But, the viruses used for gene delivery occasionally evoke severe immune responses, so scientists continue to search for non-viral delivery vehicles.
Reporting in an article to appear in the March 29 print edition of the Journal of the American Chemical Society (published on-line on March 8), the authors describe the synthesis of the new lipid molecule.
Lipid DNA complexes are attracting increasing attention as non-viral DNA delivery vehicles. They have been described as one of the "hottest new technologies" for gene therapy, accounting for nearly 10 percent of ongoing clinical trials.
Lipids are molecules with two parts, a water-liking "head group" and oily tails that assemble together to avoid water. Lipids, along with carbohydrates and proteins, constitute the main structural material of living cells.
The novel lipid molecule created at UC Santa Barbara has a tree-shaped, nanoscale head group and displays unexpectedly superior DNA-delivery properties. "It generates a honeycomb phase of lipid DNA complexes," said Cyrus R. Safinya, a professor of materials; of molecular, cellular and developmental biology; and of physics at UCSB. The new molecule was synthesized in Safinya's laboratory by first author Kai K. Ewert, a synthetic chemist who is a project scientist in the research group.
"We've been trying to get a lipid-based honeycomb lattice for a long time," said Ewert. The structure of lipid DNA complexes strongly affects their ability to deliver DNA.
"Complexes containing sheets or tubes of lipids have been known since Safinya's group found these structures in 1997 and 1998, but no one had ever seen nanoscale cylinders such as the ones in our honeycomb lattice," Ewart said. The scientists proved the formation of this novel structure with X-ray scattering experiments. Ewert designed and synthesized the new lipid by manipulating the size, shape and charge of a series of molecules. He explained that the new lipid molecule has 16 positive charges in its tree-shaped head group, the largest number by far in the field of gene delivery.
The process of delivering a gene of interest into the cell is known as "transfection." In the paper, the authors describe transfection efficiency studies carried out in four cancer cell lines using the new molecule. Two of these are mouse cell lines and two are human cell lines. The honeycomb structure turned out to be highly effective.
"Our new gene carrier shows superior transfection efficiency compared to commercially available carriers," said Ewert. "However, the most surprising result was obtained with the mouse embryonic fibroblast cells known as MEFs. These are empirically known to be extremely hard to transfect."
Safinya added: "Our data confirm that MEFs are generally hard to transfect. And the new molecule is far superior for transfection of these cells as compared to commercial lipids."
Immunological Barriers to Gene Therapy
Gene therapy has emerged as an important therapeutic tool for treatment of genetic diseases. The initial emphasis of the field for vectors for delivery of foreign genes has shifted to the immunological barriers that prevent long term transgene expressing and readministration. Extensive studies have shown that transient transgene expression is primarily due to immune responses to vector and also to the transgene product. The Immunology Core focuses on development state-of-the-art assays to assist in the understanding of mechanisms of immune-mediated transgene elimination and readministration of vectors, immune suppression/modulation regimens.

http://www.bioscience.org/
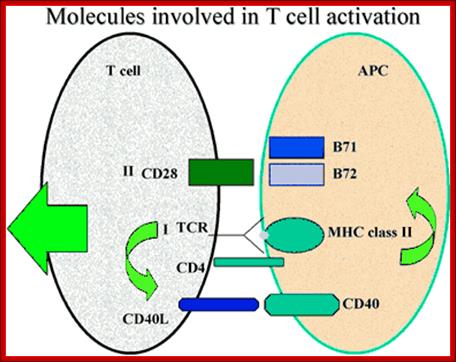
The concepts of gene therapy have opened new avenues to treatment of diseases. The central role of cellular and humoral immune responses against vector and transgene products have been appreciated since the earliest attempts of in vivo gene transfer. Several components of the immune system participate in generation of a co-ordinated cascade of events, which culminate in activation of effector cells, namely cytotoxic T cells and antibody secreting B cells. Several studies have shown that CD4 T helper cells play a central role in induction of both cytotoxic T cells and B cells. The first barrier for infections is the innate immunity, which comprises of neutrophils, tissue macrophages, NK cells, a host of soluble factors including complement, anti-microbial agents and inflammatory cytokines. Presentation of endogenous antigens by MHC class I (above) , and exogenous antigens by MHC class II (below) initiate the immune responses by activation of both CD8 and CD4 T cells. Recent advances in the mechanism of antigen presentation will allow manipulation antigen processing and presentation to interfere with activation or modulation of antigen-specific immune activation. A central role for the dendritic cells (antigen-presenting cells) in regulating immune responses has been established by the demonstration that these cells capture and process antigens, express lymphocyte co-stimulatory molecules, migrate to lymphoid organs and secrete cytokines.

http://www.bioscience.org/
Activation of CD4+ T cells (below) requires two signals; one transduced by recognition of antigen in the context of MHC class II molecules, and the second by co-stimulatory molecules e.g. CD28. Several studies have suggested that interaction of CD40L on T cells with CD40 on antigen presenting cells leads to expression of CD80/CD86 molecules which in turn bind to CD28 on T cells. Interruption of this sequential cascade of events results in T cell unresponsiveness. Delineation of critical events leading to complete T cell effector function has allowed development of novel strategies in regulation of immune responses, which may ultimately lead to long term transgene expression following gene therapy.
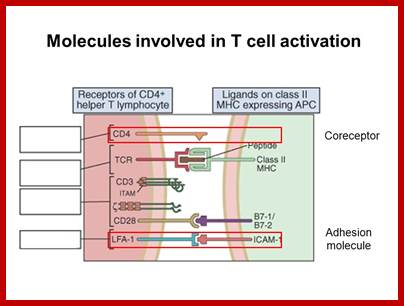
http://slideplayer.com/
Regulated activation of B cells also involves a complex series of events leading to immunoglobulin secretion, which neutralizes soluble transgene expression, and impedes re-administration potential of viral vectors. Antigen is first complexed by components of complement systems and then presented to B cells in complex form on follicular dendritic cells in the lymph nodes. The complexed antigen is taken up by B cells through the BCR and processed and presented by MHC class II to T helper cells. Activated B cells expressing CD80/CD86 trigger activation of CD4 helper T cells. The helper function provided by CD4+ T cells to induce B cells to differentiate in to immunoglobulin secretion includes cell - cell contact, through molecular interactions, e.g. CD40L - CD40, or soluble cytokines, e.g. IL-4. It is in these responses that B cells generate immunological memory, and increase affinity by somatic mutation and finally differentiate into antibody secreting plasma cells.
A majority of the pharmaceutical intervention strategies that have been extensively investigated in clinical trials for transplantation and are being adapted for gene therapy are inhibitors of cell mediated immune function. With the advent of novel vectors, e.g. AAV and lenti-viruses which result in stable gene expression over prolonged periods for the first time in treatment of human disease, immuno suppressive therapies need to be targeted to inhibition of antigen-specific B cells.
There many therapies have been employed so far. They are- surgery, Chemotherapy, Radiation therapy, Targeted therapy, Hypothermia therapy, Stem cell transplant therapy, Photodynamic therapy, Laser treatment; In 2016 IISc scientist Dr. Chakravarty used what is called photodynamic therapy using Vanadium bound to DNA; Med Chem Comm, Royal society of chemistry, united kingdom.
Cancer- immunotherapy 2106:
Scanning electron micrograph of a human T lymphocyte (also called a T cell) from the immune system of a healthy donor. Source: National Institute of Allergy and Infectious Diseases (NIAID)
A healthy T cell; Credit Flicker/CC BY 2.0; https://www.cancer.gov
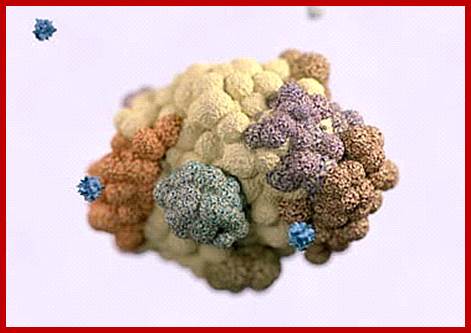
Cancer Cells- in the form of Tumor. http://www.cancer.org/
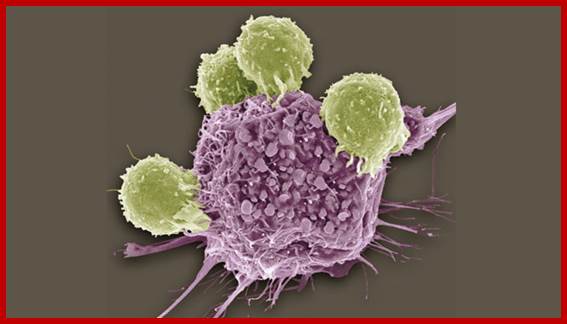
T CELLS ATTACK: Colored scanning electron micrograph (SEM) of T lymphocytes (green) bound to antigens on a cancer cell. Can researchers harness the killing power of patients’ own immune systems to treat cancer?© STEVE GSCHMEISSNER/SCIENCE SOURCE; http://www.the-scientist.com
Types of cancer immunotherapy: The main types of immunotherapy now being used to treat cancer include: • Monoclonal antibodies: These are man-made versions of immune system proteins. Antibodies can be very useful in treating cancer because they can be designed to attack a very specific part of a cancer cell. • Immune checkpoint inhibitors: These drugs basically take the ‘brakes’ off the immune system, which helps it recognize and attack cancer cells. • Cancer vaccines: Vaccines are substances put into the body to start an immune response against certain diseases. We usually think of them as being given to healthy people to help prevent infections. But some vaccines can help prevent or treat cancer. • Other, non-specific immunotherapies: These treatments boost the immune system in a general way, but this can still help the immune system attack cancer cells. Immunotherapy drugs are now used to treat many different types of cancer. For more information about immunotherapy as a treatment for a specific cancer, please see our information on that type of cancer. Many newer types of immunotherapy are now being studied for use against cancer. Some of these are discussed in “What’s new in cancer immunotherapy research?” http://www.cancer.org/
Treatment; Immune Cell Therapy
Progress is also being made with an experimental form of immunotherapy called adoptive cell transfer (ACT). In several small clinical trials testing ACT, some patients with very advanced cancer—primarily blood cancers—have had their disease completely eradicated. In some cases, these treatment responses have lasted for years.
In one form of ACT, T cells that have infiltrated a patient’s tumor, called tumor-infiltrating lymphocytes (TILs), are collected from samples of the tumor. TILs that show the greatest recognition of the patient's tumor cells in laboratory tests are selected, and large populations of these cells are grown in the laboratory. The cells are then activated by treatment with immune system signaling proteins called cytokines and infused into the patient’s bloodstream.
The idea behind this approach is that the TILs have already shown the ability to target tumor cells, but there may not be enough of them within the tumor microenvironment to eradicate the tumor or overcome the immune suppressive signals that are being released there. Introducing massive amounts of activated TILs can help to overcome these barriers and shrink or destroy tumors.
Use of modified T cell therapy; http://www.healthline.com/ http://www.cbsnews.com/
Programmed Cell Death- making tumour implode
:

Modified killer T lymphocyte approaches a cancer cells and attcks attacks cancer cells shown below http://www.healthline.com/ http://www.cbsnews.com/
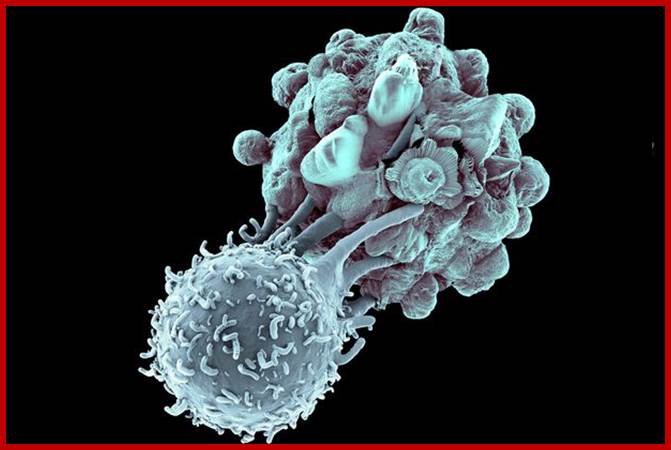
T-cell immunotherapy is created from T-cells bottom left in left image)
– white blood cells that normally fight off viruses and bacteria. These are
removed from the patient and genetically tweaked to recognise and attack their
cancer. The genetically-modified cells are then grown in their millions in a
lab before being infused back into the patient, where they hunt down cancer
cells (right)
Follow us: @MailOnline on Twitter | DailyMail on Facebook;A modified T-cell attacks a cancer cells; http://www.dailymail.co.uk

The ‘extraordinary’ results are ‘unprecedented in medicine’, the world’s
biggest science conference heard
Read more: http://www.dailymail.co.uk/health/article-3448598/The-living-drug-wipe-cancer-Stunning-new-therapy-hunts-destroys-diseased-cells-prevents-returning.html#ixzz4h6TjMwA4
Follow us: @MailOnline on Twitter | DailyMail on Facebook;http://www.dailymail.co.uk
CAR-Cell therapy:
Another form of ACT that is being actively studied is CAR T-cell therapy. In this treatment approach, a patient’s Cells were collected from the blood and genetically modified to express a protein known as a Chimeric Antigen Receptor, or CAR. Next, the modified cells are grown in the laboratory to produce large populations of the cells, which are then infused into the patient. CARs are modified forms of a protein called a T-cell receptor, which is expressed on the surface of T cells. These receptors allow the modified T cells to attach to specific proteins on the surface of cancer cells. Once bound to the cancer cells, the modified T cells become activated and attack the cancer cells. Over the last decade, targeted therapies like imatinib (Gleevec®) and trastuzumab (Herceptin®)—drugs that target cancer cells by homing in on specific molecular changes seen primarily in those cells—have also emerged as standard treatments for a number of cancers.

Cancer cells and T cells; http://cancerprogress.net/
Molecular Target therapy:
It is called “molecularly targeted therapy.” The treatment consists of drugs designed at the molecular level of the cell to specifically attack and kill only the cancer cells of a specific type of cancer. And they are tailor-made to recognize specific molecules unique to specific cancers.
The model drug leading the way is Glivec, also known as STI571. It is active against a relatively rare form of leukaemia — chronic myeloid leukaemia, or CML — characterized by excessive overproduction of white blood cells. Approximately 7,000 Americans are diagnosed with CML each year. http://abcnews.go.com/
|
Table 1. Characteristic infections of the primary immunodeficiencies |
|||
|
component |
primary pathogen |
primary site |
clinical example |
|
T-cells |
intracellular, bacteria viruses, protozoa, fungi, |
non-specific |
SCID, DiGeorge |
|
B-cells |
pneumococcus, streptococcus, haemophilus |
lung, skin, CNS |
IgG, IgM deficiency IgG, IgM deficiency |
|
enteric bacteria and viruses |
GI, nasal, eye |
IgA deficiency |
|
|
phagocytes |
Staphylococcal, Klebsiella Pseudomonas, |
lung, skin, regional lymph node |
chronic granulomatous disease (CGD) |
|
complement |
Neisseria, Haemophilus, pneumococcus, streptococcus |
CNS |
C3, Factors I and H, late C components |
.
|
Table 2. Summary of T cell and B cell immunodeficiency diseases (ID) |
|||||||
|
Disease |
T-cells |
B-cells |
Immunoglobulins |
Inheritance |
|||
|
No. |
Fx |
IgM |
IgG |
IgA |
|||
|
Reticular dysgenesis |
A |
A |
A |
A |
A |
A |
u |
|
CID (autosomal) |
A/L |
A/L |
A/L |
A/L |
A/L |
A/L |
a |
|
SCID (x-linked) |
A/L |
A/L |
A/L |
A/L |
A/L |
A/L |
x |
|
DiGeorge's syndrome |
A/L |
A/L |
N/V |
N/V |
N/V |
N/V |
a/x |
|
ataxia telangiectasia |
L |
L |
L |
N/V |
L/V |
L |
a |
|
Wiskott-Aldrich |
?V |
L |
L/V |
L |
N |
H |
x |
|
also high IgE |
|||||||
|
x-linked hypo-gamma- globulinemia |
N |
N |
L |
L |
L |
L |
x |
|
selective IgA ID |
N |
N |
N |
N |
L/V |
L |
a/x |
|
hyper-IgM hypo-gamma- globulinemia |
N |
N |
N |
H |
L |
L |
x |
|
transient hypo-gamma- globulinemia |
N |
N |
N |
N |
L |
L |
a? |
|
common variable hypo-gamma- globulinemia (teens-adult) |
N |
N |
N |
N |
L |
L |
none |
|
A: absent; a: autosomal; H: high; L: low; N: normal; U; unknown; V: variable; x: x-linked |
|||||||
|
|
Immunotherapy 2016: