Immunology5
B and T cell activation And Immunity.
Stem cells: Colored scanning electron micrograph (SEM) of stem cells taken from umbilical cord blood. These cells are known as multipotent because they undergo differentiation to produce precursors of all the body’s specialized blood cells. By this process, termed hematopoiesis, stem cells develop either into red blood cells, or one of several types of white blood cells that make up the immune system. The purification of stem cells from umbilical cord blood allows scientists to research the function of the immune system and to develop treatments for diseases such as AIDS and leukemia. http://mrc.iisc.ernet.in/
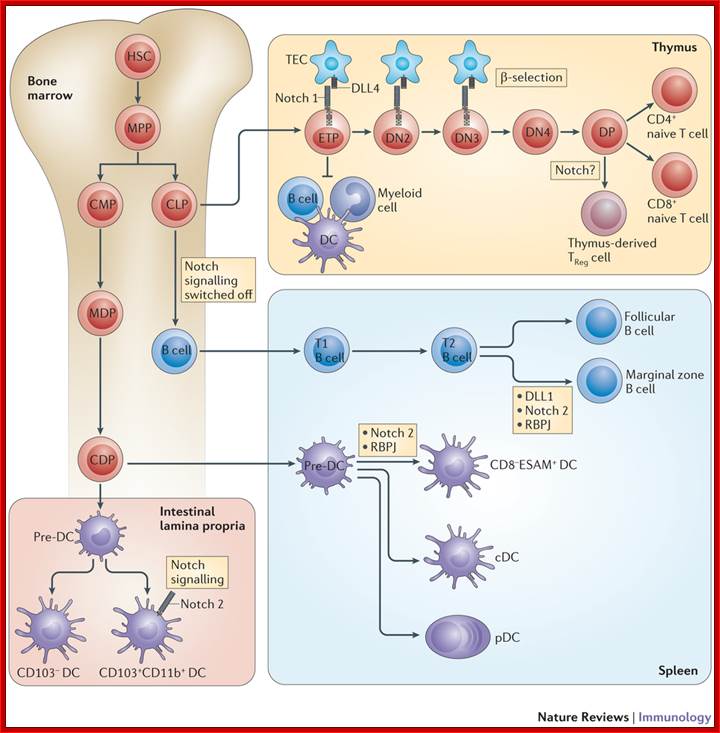
Notch signaling in immunocell development: Bone marrow hematopoietic stem cells (HSCs) give rise to multipotent progenitors (MPPs) before differentiating into common myeloid progenitors (CMPs) and common lymphoid progenitors (CLPs). CLPs migrate from the bone marrow to the thymus, where thymic epithelial cells that express Delta-like ligand 4 (DLL4) trigger canonical Notch 1 signalling in early thymic progenitors (ETPs). This Notch 1 signal is essential for T cell lineage commitment and is further required during early phases of thymocyte differentiation up to the double-negative 3 (DN3) stage. Active Notch signalling during these early stages of T cell development inhibits other lineage potentials, such as B cell and myeloid cell (including dendritic cell (DC)) potential. During β-selection, Notch signalling is turned off as a consequence of pre-T cell receptor signalling. Thus subsequent stages of T cell development exhibit very low levels of Notch signalling. Notch was also suggested to influence the development of regulatory T (TReg) cells (specifically, thymic TReg cells). In bone marrow-residing CLPs, Notch signalling must be switched off to allow proper B cell development. After migration of immature B cells to the spleen, interaction of DLL1 with Notch 2 (mediated by recombination signal binding protein for immunoglobulin κJ region (RBPJ)) induces Notch signalling in transitional B (T2) cells to specify marginal zone B cells as opposed to follicular B cells. The vast majority of DCs are derived from CMPs in the bone marrow, which give rise to macrophage–DC progenitors (MDPs). Subsequently, common DC progenitors (CDPs) develop into pre-DCs, seeding lymphoid and non-lymphoid organs via the bloodstream. In the spleen these pre-DCs are specified into multiple DC subsets, including classical DCs (cDCs) and plasmacytoid DCs (pDCs). Splenic CD8− endothelial cell-selective adhesion molecule (ESAM)+ DCs and CD103+CD11b+ DCs in the lamina propria of the intestine require Notch signalling mediated by the Notch 2 receptor. DP, double-positive; TEC, thymic epithelial cell; Freddy Radtke, H. Robson MacDonald & Fabienne Tacchini-Cottier
http://www.nature.com/

Among the stem cells (multipotent or pleuripotent) some undergo differentiation and development into B cells and some into T cells and other white blood cells. http://www.nature.com/
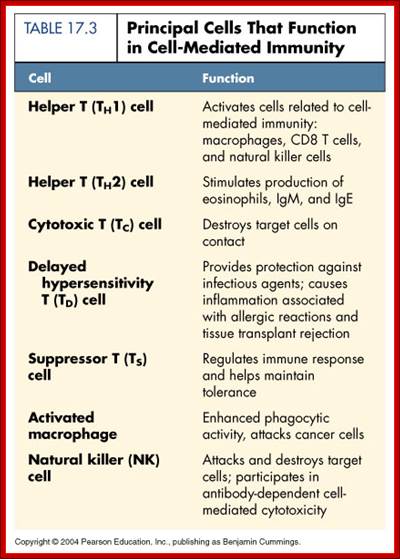
Principal Cells that function in Cell mediated Immunity;
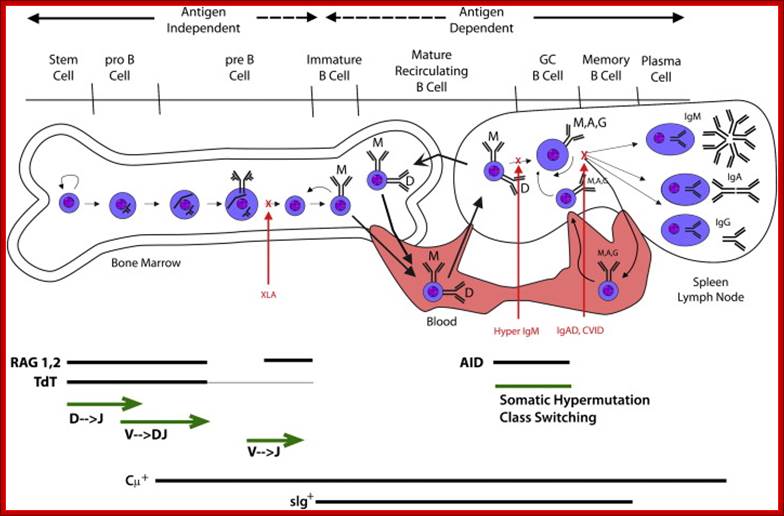
Activated B cells in response to the binding of antigens directly or through T helper cells the genes for their immunoglobulin go through diversification through a remarkable somatic genetic recombination diversification. This goes hand in hand with differentiation and development of B. B-cell development as a function of immunoglobulin rearrangement and modification is shown. After birth, B-cell development begins in the bone marrow and is independent of antigen stimulation. The pre–B cell is defined by the presence of cytoplasmic μ protein (Cμ+). With development, the fate of the B cell becomes increasingly dependent on its response to antigen. Immature B cells leave the bone marrow and begin to express IgD. They recirculate through the blood, the secondary lymphoid organs, and the bone marrow. Encounter with cognate antigen can cause the cell to become a memory B cell or a plasma cell. Patients with X-linked agammaglobulinemia (XLA) lack Burton’s tyrosine kinase function and have difficulty making immature B cells and IgM. Patients with hyper-IgM syndrome (Hyper IgM) are unable to class-switch. Patients with selective IgA deficiency (IgAD) or common variable immune deficiency (CVID) can class-switch but have difficulty becoming plasma cells or memory B cells. www.keywordsking.com
B cell receptors (BCRs), Activation and Antibody synthesis:
In humoral immune response, B cells are activated and secrete Igs that act to eliminate antigens. Both protein and non-protein antigens can stimulate antibody production. B cell responses to protein antigens require CD4 helper T cells specific for antigens.
Schematic of a possible mechanism of action of antibody-independent cell-mediated immunity.
Towards a blood-stage vaccine for malaria: are we following all the leads?
Michael F. Good

The dendritic cell presents a peptide antigen, bound to an MHC class II molecule, to the CD4+ lymphocyte. The peptide-MHC complex is recognized by the T cell receptor complex. A co-stimulatory signal, delivered via CD80 or CD86, is necessary for lymphocyte activation. CD28 acts as a positive co-stimulator and CTLA-4 is a negative co-stimulator in this context. Soluble CTLA-4 regulates lymphocytic activation by competing with CD28 for binding to CD80 or CD86. The interaction between PD-L1 and PD-CD1 interaction inhibits T cell activation, as does the action of LYP to dephosphorylate Fyn and Lck. Abbreviations: CTLA-4, cytotoxic T lymphocyte antigen-4; Fyn, tyrosine-protein kinases; Lck, tyrosine-protein kinases; LYP, lymphoid tyrosine-protein phosphatase non-receptor type 22; PD-1, programmed cell death protein 1; PD-L1, programmed cell death 1 ligand 1; TCR, T cell receptor. http://www.nature.com
Some of the factors that are thought to give rise to
antibody-independent (T-cell) immunity are shown. This commences with the
activation of CD4+ T cells by mature dendritic cells leading to
macrophage activation, phagocytosis of parasitized red blood cells (pRBC), and
elaboration of cytokines and small inflammatory molecules (such as nitric oxide
and oxygen radicals). T-cell immunity is thought to occur largely, but not
entirely, in the spleen; IFN-![]() , interferon-
, interferon-![]() ; IL-12, interleukin-12; MHC II, major histocompatibility complex
class II; TCR, T-cell receptor; and TNF alpha.
; IL-12, interleukin-12; MHC II, major histocompatibility complex
class II; TCR, T-cell receptor; and TNF alpha.
B cell activation is initiated by clustering of antigen receptor (membrane IgM and IgD on naïve B cells) by binding of multivalent antigens. Membrane Ig associated signaling molecules Ig alpha and Ig beta transduce signals on antigen binding to Ig and these signals lead to the activation of transcription factors and expression of various genes.
Helper T cell dependent B cell responses to protein antigen require initial activation of naïve T cells in the T cell zone and of B cells in lymphoid follicles in lymphoid organs. The activated lymphoid cells migrate towards one another and interact at the edges of follicles, where the B cells present the antigens to helper T cells.
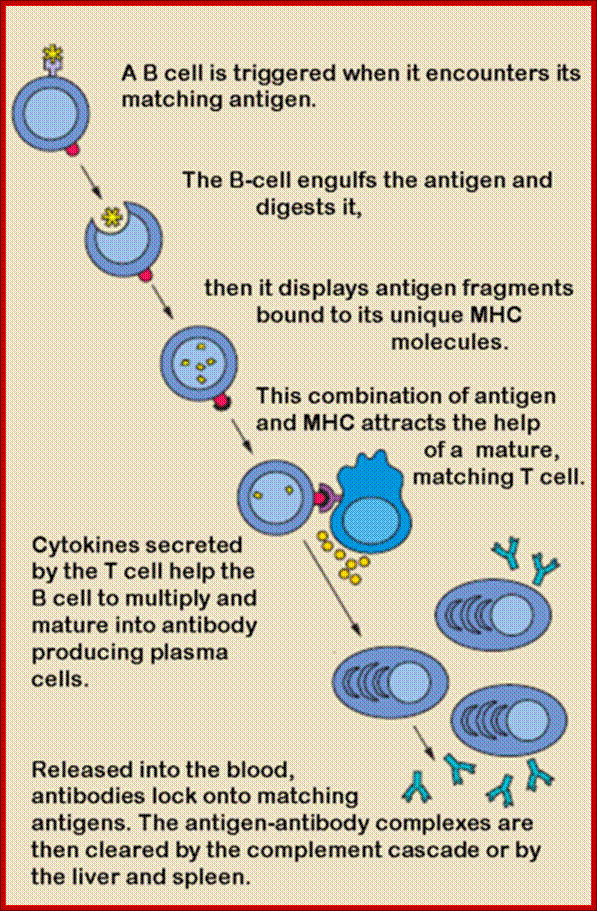
Activated helper T cells express CD40L, which engages CD40 on B cells and T cells secrete cytokines that bind to cytokine receptors on B cells. The combinations of CD40 and cytokines signals stimulate B cell proliferation and differentiation into antibody producing and secreting cells.
Helper T cell derived signals including CD40L and cytokines also induce isotype switching in B cells by a process called switch recombination, leading to production of Ig various isotypes. Different isotypes mediate different effector functions.
Germinal centers are formed inside follicles of spleen and lymph nodes when activated B cells migrate into follicles and proliferate. The late events in T cell-dependent antibody responses including affinity maturation and generation of memory cells takes place within germinal centers.
The cellular and humoral adaptive immune system generates a remarkable breadth of diversity of antigen receptors by combinatorial shuffling in of gene segments in somatic cells. The diversity of possible receptors is large and until recently this diversity precluded the possibility of capturing the antigen receptor repertoires. We have developed a method that amplifies rearranged T-cell and B-cell receptor variable regions and uses high-throughput sequencing to capture millions of these sequences. We have applied the technology to a variety of clinical problems including MRD detection in hematological malignancies, patient stratification for immunotherapy, measuring immune reconstitution after transplant, and characterizing TIL populations; Autoreactive B-cells. http://test.biomedicalresearchcentre.org/
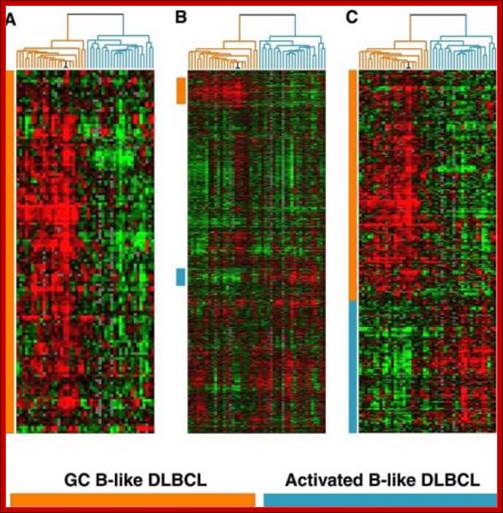
Activated B cell gene expression microarray during development; Alizadeh. Nature, 2000. http://www.acbd.monash.org/
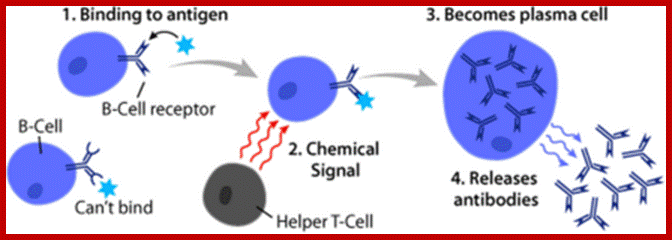
Activation of B-cell by an antigen leading to memory and plasma cells;
B-cell progenitor
http://www.wikiwand.com

After initially binding an antigen to the B cell receptor (BCR), a B cell internalizes the antigen and presents it on MHC II. A helper T cell recognizes the MHC II–antigen complex and activates the B cell. As a result, memory B cells and plasma cells are made; https://cnx.org
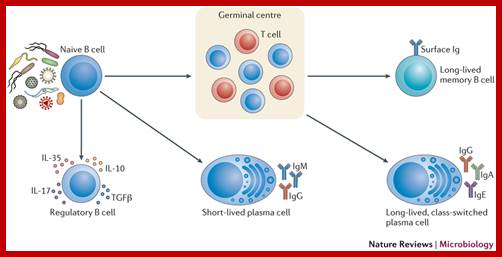
In response to activation signals, naive mature B cells proliferate and differentiate into effector cells. B cell activation results from the integration of several infection-related signals, including binding of specific antigens to the B cell receptor (BCR) and pattern recognition receptor (PRR) ligands46. In an early polyclonal response, short-lived plasma cells that secrete polyreactive antibodies can be generated7. Regulatory B cells can also be induced and exert an immunosuppressive function by secretion of interleukin-10 (IL-10), IL-17, IL-35 and transforming growth factor-β (TGFβ), which modulate T cell responses. Sustained B cell activation leads to further differentiation and selection in organized lymphoid structures, called germinal centres (GCs). This occurs through cytokine signalling and the interaction between CD40 on B cells and CD40 ligand on cognate T cells (not shown). The activation of nuclear factor-κB (NF-κB) and upregulation of activation-induced cytidine deaminase (AID) induce affinity maturation of antibodies through somatic hypermutation and class-switch recombination of the antibody heavy chain. This ultimately results in the differentiation of specific, long-lived plasma cells and memory B cells, which confer protective immunity. Ig, immunoglobulin. Armelle Phalipon, et al; http://www.nature.com

B-Plasma cell; http://www.biolegend.com
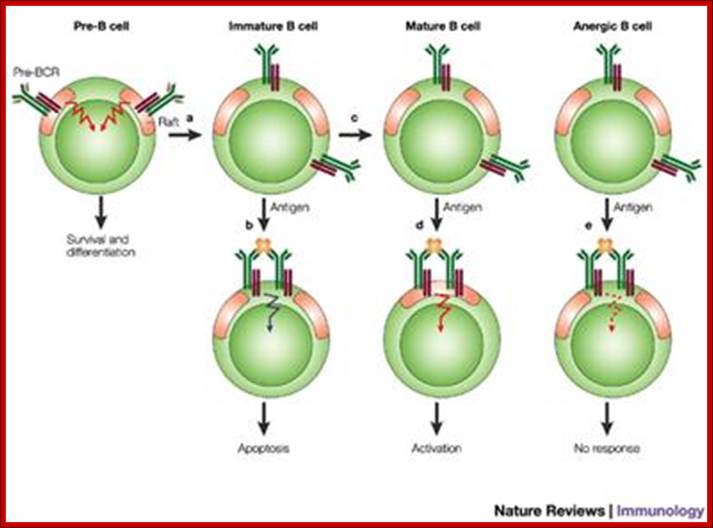
The B-cell receptor (BCR) and rafts in B-cell development: lipid rafts and B cell activation; In pre-B cells, the pre-BCR resides constitutively in lipid rafts and signals for light-chain rearrangement and continued development to the immature B-cell stage (a). In immature B cells, the BCR is excluded from rafts even after antigen binding that leads to apoptosis (b). If antigen is not encountered by the immature B cell, development continues to the mature B-cell stage (c). In mature B cells, antigen binding leads to association of the BCR with rafts and to B-cell activation (d). The BCR expressed by B cells that have been made tolerant or anergeric as a result of chronic exposure to antigen is excluded from lipid rafts even after antigen binding, which fails to activate the cells (e). Pre-B to Mature B-cell and other forms; Susan K. Pierce; http://www.nature.com
B-cells in bone marrow develop as stem cells and differentiate into T-cells and B-cells. While T-cell maturation into active cells takes place in Thymus (where they are called Thymocytes, B-cell continue to differentiation and develop a repertoire of IgM that stick to their membranes providing a diverse array of antigen receptors at least 10^9 kinds and they circulate in blood vessels and lymphocytes.
When they encounter an antigen, it can be viral protein, a bacterial cell wall materials, or lipopolysaccharides, they get activated, leading to the phagocytosis of the bound antigen, which is then processed and into small peptides and loaded on to MHC APC presenters and the same is presented on its cell surface. It is at this point T-cells recognize, depending upon the type of MHC, and bind. This leads B-cells to go through activation its specific genes and express the same AB and released into blood stream. While this process takes place when the activated B-cell undergoing proliferation. Among the active proliferated cells some of them move into BM and reside as memory cells retaining the membrane bound antigen receptors for future use.
B cell releasing Cytokines; https://www.slideshare.ne
http://www.keyword-suggestions.com
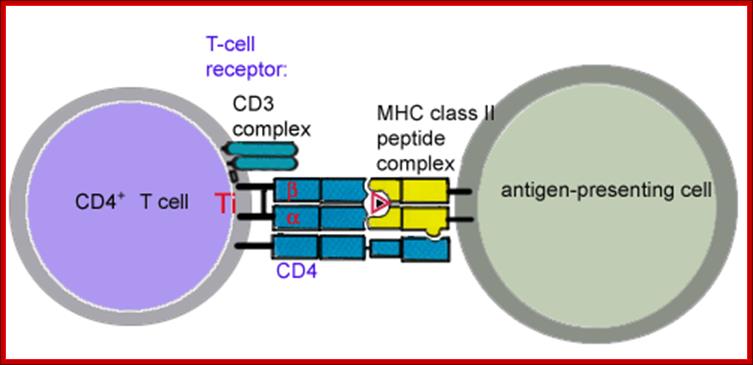
CD8+ T cells play a role in the elimination of virally infected cells. The infected cell marks itself as a target for the cytotoxic T cell by displaying peptides derived from intracellular viral protein on its surface. Viral proteins are bound to peptide-binding regions of class I MHC molecules. Peptides which bind to MHC Class I molecules come from proteins synthesized within the cell (which are broken down) and transported to the endoplasmic reticulum.
B cells: the earliest cells which develop are called B1 cells; they express CD5 cell-surface molecule and are the source of "natural antibodies", which are IgM antibodies and are frequently polyreactive (recognize different antigens, pathogens and auto-antigens). Natural antibodies have a relative low affinity. Most B cells lack the CD5 molecule; they develop later and are called B2 cells. Mature B2 cells coexpress IgM and IgD antibodies on their cell surface. The genes encoding B-cell receptors undergo a process of somatic hypermutation; the final stages of differentiation of B2 cells into antibody secreting plasma cells occurs within the germinal centers of secondary lymphoid tissues. To elicit a strong antibody response, B cells require antigens, T cells for direct contact (usually TH2 Cells), soluble cytokines (IL4+Il13, INF-y or Il10, Certain adhesion molecules. CD4 T cell locks on to antigen containing MHC class II proteins and activates B cells and also T cells themselves; http://www.medicine.mcgill.ca/
Lymphocyte activation, maturation and regulation of Lymphocytes:
Bone marrow is the site for B and T cell origin. B/lc mature in B/M and T cell progenitors migrate to Thymus and there they mature. Early maturation by cell proliferation activated by cytokines mainly by il7 leading to increase in mature lymphocytes.
B and T cell maturation involves somatic recombination of antigen receptor gene segments and the expression Ig molecule such as BCR and TCR on B and T cells respectively. This expression provides a repertoire of useful antigen specificities.
T and B cell receptor genes are spatially segregated in germ line antigen loci. They contain separate loci for Ig heavy and Ig kappa light, and Ig lambda chains, TCR beta and TCR alpha and gamma and TCR delta. These loci contain V, J in Ig H chains and TCR beta and delta loci D gene segments. Somatic recombination of both Ig and TCR loci involves the joining of D and J segments in the loci that contains D segments ,Followed by the joining of V to recombined DJ in these loci or direct V to J joining in other loci. This recombination is facilitated by recombinase enzyme complex that includes lymphocyte-specific components RAG1 and RAG2.
The diversity of BCR and TCR repertoire is generated by the combinatorial associations of multiple germ line V,D and J genes and junction diversity generated by the addition of random nucleotides to the sites of recombination These mechanisms generate maximum diversity at the junctions of V and C regions that form the third hyper variable region of both antibody (BCR) and TCR polypeptides.
B cell saturation occurs in stages characterized by different pattern of Ig gene recombination and expression. In the earliest B cells called pro-B Ig genes exist in germ line configuration. In the pre B cell stage, VD-J recombination occurs in the Ig-H locus. The primary RNA transcript containing VDj complex and IgG gene segments are produced and the micro C region exons of the heavy chain RNA are spliced to the VDJ segment to generate a mature RNA that is translated into mature micro heavy chain protein. A pre BCR is formed by pairing of micron chain with nonvariable surrogate light chain and by association with the signaling molecules Ig alpha and Ig Beta. This receptor mediates signals that inhibit rearrangement on the other heavy chain alleles (allelic exclusion) as well as stimulate proliferation. In the immature B-cell stage V-J recombination occurs in the K or lambda loci and the light chain proteins are expressed. H and L chain proteins are assembled into intact IgM and expressed on the cell surface. Immature B cells leave bone marrow to populate peripheral lymphoid tissue, where they complete their maturation. In mature B cell stage, synthesis of both micron and delta heavy chains also occur in the same B cell. Mediated by alternative splicing of primary heavy chain RNA and membrane IgM and IgD are expressed.
During B cell maturation, selection process eliminates or inactivate B cell precursor that express high affinity antigens receptors specific for self-antigens present in the Bone marrow.
The B-cell receptor is composed of two parts:
- Ligand binding moiety: A membrane-bound immunoglobulin molecule of one isotype (IgD, IgM, IgA or IgE). With the exception of the presence of an integral membrane domain, these are identical to their secreted forms.
- Signal transduction moiety: A heterodimer called Ig-α/Ig-β (CD79), bound together by disulfide bridges. Each member of the dimer spans the plasma membrane and has a cytoplasmic tail bearing an immunoreceptor tyrosine-based activation motif (ITAM).
B-Cell Signal Transduction: Tyrosine Phosphorylation, Kinase Activity, and Calcium Mobilization.
Tilman Brummer, Winfried Elis, Michael Reth, and Michael Huber
Signal transduction by the B-cell antigen receptor (BCR) regulates development, survival, and clonal expansion of B cells. The BCR complex comprises the membrane-bound immunoglobulin molecule and the Ig-α/Ig-β heterodimer, and was shown to form oligomeric structures. Antigen mediated engagement of the BCR results in the tyrosine phosphorylation of multiple signaling proteins leading to calcium mobilization and the activation of downstream serine/threonine kinases as well as transcription factors. In pervanadate (PV)-treated B cells, comparable pathways are activated on expression of the BCR, indicating that the BCR can signal in an antigen-independent fashion as well. In this chapter, we describe the analysis of antigen-dependent and –independent tyrosine phosphorylation events as well as a method to study calcium mobilization from differentially stimulated B cells. Furthermore, we emphasize the use of phospho-specific antibodies (Abs) and low-molecular-weight enzyme inhibitors in the process of mapping BCR-activated signaling pathways as well as determining activation states of signaling proteins.
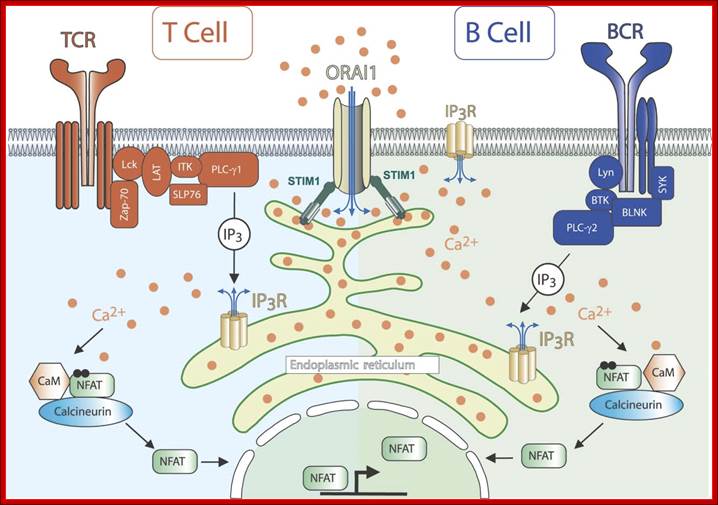
Calcium ions regulate almost all cellular processes. Inositol 1,4,5‐trisphosphate ; com receptors (IP3Rs) are ubiquitous calcium channels which mediate calcium release primarily from endoplasmic reticulum stores. IP3Rs are activated by the soluble second messenger inositol 1,4,5‐trisphosphate (IP3), which is produced in response to various stimuli by the action of phospholipase C enzymes. Very soon after the discovery that IP3 is a calcium mobilizing second messenger, it was found that T‐cell receptor signaling is dependent upon IP3R activity. It is now known that cell signaling throughout the immune system is dependent upon IP3R‐dependent calcium release for many critical processes, including cellular activation, proliferation, and death. This channel plays a critical role in orchestrating the immune response in health and disease and thus offers potential as a therapeutic target in immune‐related disorders. WIREs Membr Transp Signal 2012,1:329–339. doi: 10.1002/wmts.27; http://www.keywordsking. https://fmhusmp.blogspot.com
Signals through the pre-BCR are required for initiating diverse processes in pre-B cells, including proliferation and recombination of the light chain gene, which eventually lead to the differentiation of pre-B cells to immature B cells. However, the molecular mechanisms by which the pre-BCR promotes these processes remain largely unresolved. Recent findings suggest that fork head box O (FOXO) transcription factors connect pre-BCR signaling to the activation of the recombination machinery. In this Review, we discuss how FOXO transcription factors are regulated by the pre-BCR to allow the progression of the cell cycle and the recombination of the light chain gene.
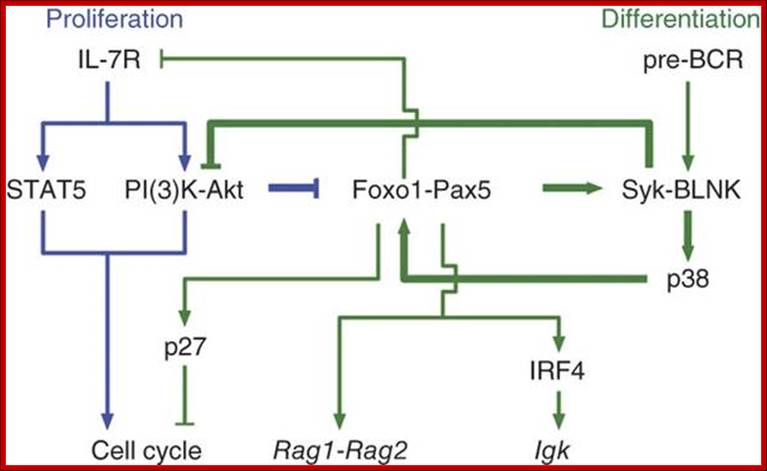
The molecular crosstalk between the interleukin 7 receptor (IL-7R) and the precursor to the B cell antigen receptor (pre-BCR) in B lymphopoiesis has not been elucidated. Here we demonstrate that in pre-B cells, the IL-7R but not the pre-BCR was coupled to phosphatidylinositol-3-OH kinase (PI(3)K) and the kinase Akt; signaling by this pathway inhibited expression of recombination-activating gene 1 (Rag1) and Rag2. Attenuation of IL-7 signaling resulted in upregulation of the transcription factors Foxo1 and Pax5, which coactivated many pre-B cell genes, including Rag1, Rag2 and Blnk. Induction of Blnk (which encodes the signaling adaptor BLNK) enabled pre-BCR signaling via the signaling molecule Syk and promoted immunoglobulin light-chain rearrangement. BLNK expression also antagonized Akt activation, thereby augmenting the accumulation of Foxo1 and Pax5. This self-reinforcing molecular circuit seemed to sense limiting concentrations (Kyoko Ochiai,1 et al 2012)
The response of B cells to the binding of antigens and the binding of T helper cell leads, first signal transduction. This leads various other activities such as engulfment of the antigens, digestion, processing and projection of antigens via MHC class II on its cell surface. With antigen projected on the surface of B cells T helper cells recognize MHC class II antigens and binds that leads to another signal transduction and production of specific gene recombination and release of fully formed IgG into cytoplasm. It is followed by synthesis and presentation of CD40L (=CD154) on the Th2 cell, which binds to CD40 on the B cell, thus the Th2 cell can co-stimulate the B cell. Without this co-stimulation the B cell cannot proliferate further.
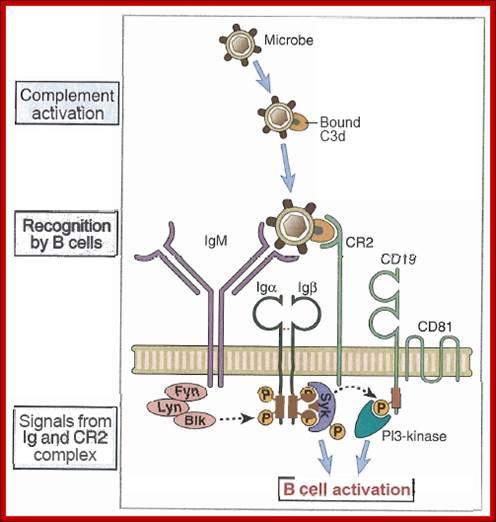
Co-stimulation for B cells is provided alternatively by complement receptors. Microbes may activate the complement system directly and complement component C3b binds to microbes. B cells express complement receptor CR2 (CD21) to bind to iC3b, C3dg, or C3d. This additional binding makes the B cells 100- to 10,000-fold more sensitive to antigen.
Although B cells do not need antigen presentation to be activated, binding of BCR to an array of identical epitopes bound to antibody on Fc receptors (FcR) on macrophages and neutrophils efficiently stimulate B cell activation.
The signaling process is due their receptors having cytoplasmic domains which are in contact with other complexes such as protein kinases. The binding of antigens activates the protein kinases that are bound to cytoplasmic domain of the BCR. The protein kinases are tyrosine or serine specific found as transmembrane components. An important signaling molecule which binds phosphorylated membrane molecules is phospholipase C (PLC). PLC is an enzyme that cuts the membrane phospholipid phosphatidylinositol biphosphate (PIP2) into two other signaling molecules, inositol triphosphate (IP3) and diacylglycerol (DAG). IP3 diffuses away from the membrane and releases calcium from intracellular storage sites. Increased calcium levels activate the calcium-binding molecule calmodulin, which binds and activates other signaling molecules. DAG remains associated with the inner side of the plasma membrane and (with calcium) activates protein kinase C (PKC), which phosphorylates still other signaling molecules. This cascade of enzyme activations rapidly transmits the membrane binding signal through the cytoplasm. Both antigen and growth factors signal lymphocytes to divide. In order for the signaling molecules to bind different receptors, adaptor proteins are needed. Adaptor proteins are not signaling molecules, but they connect the signaling enzymes with their substrates. Small G proteins also link receptor tyrosine phosphorylation to cytoplasmic kinases. Small G proteins like Ras are inactive when they bind GDP and active when they bind GTP. Since small G proteins have phosphatase activity that converts GTP to GDP, they are normally inactive. Adaptor proteins bind small G proteins to the membrane receptors. Receptor-activated factors (GEFs) then displace GDP and allow GTP to bind and activate the small G protein. The small G protein then activates a cascade of protein kinases called MAP kinases, which phosphorylate transcription factors in the nucleus. Transcription factors bind the promoter region of the DNA preceding the coding region and promote RNA polymerase binding and synthesis on mRNA. Both CD3 and IgIg have much longer cytoplasmic domains than BCR and TCR. They also have specific amino acid sequences, ITAMs (Immunoreceptor Tyrosine-based Activation Motifs), which become phosphorylated by receptor-associated tyrosine kinases.
When the antigen receptors cluster after binding antigen, src-family protein tyrosine kinases (PTKs) phosphorylate the ITAMS on the IgIg or the CD3. The activity of the src-family PTKs are regulated by two other protein kinases. Activation depends on CD45 (leukocyte common antigen) which is required for receptor-mediated activation of lymphocytes.
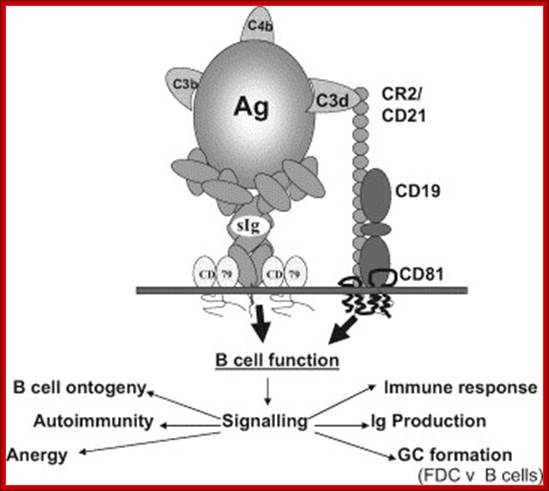
The CR2–BCR complex; The cartoon illustrates the structure of the complex between CR2 and the B cell receptor (BCR), with recruitment of additional signaling components to generate a signalling competent complex. Binding of C fragment opsonized immune complex (Ag here) to the BCR co-ligates CR2 in the complex, providing additional signals and hence increased response in the B cell. https://www.slideshare.ne
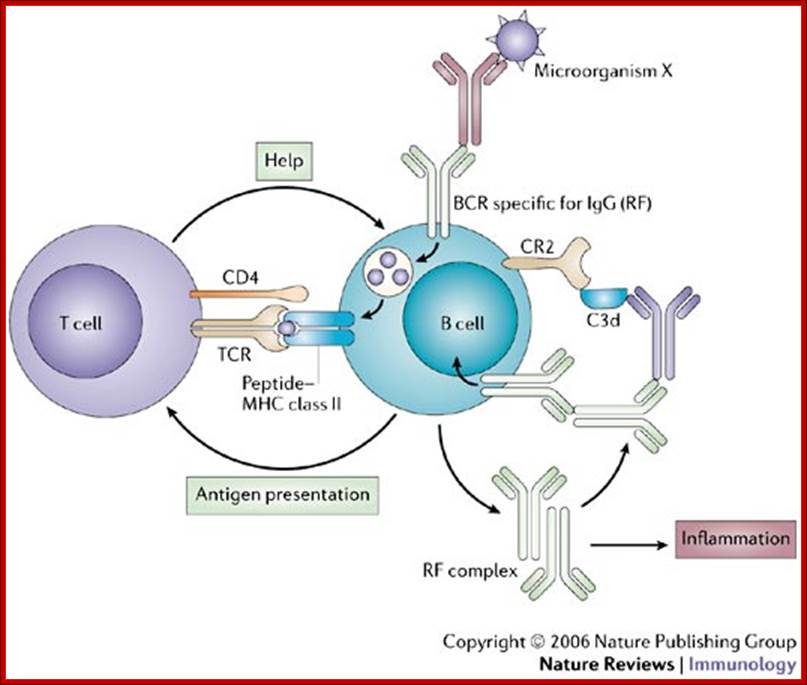
Model for self-perpetuation of auto-reactive B cells;
IgG rheumatoid factor (RF)-secreting B cells provide the simplest example of how auto-reactive B cells might become 'self-perpetuating'. A B-cell receptor (BCR) specific for IgG (RF) can mediate the endocytosis of foreign antigens and acquire help from T cells specific for such antigens following antigen presentation. Soluble IgG RF can polymerize and acquire complement component 3d (C3d), which can amplify re-afferent (feedback) signals through BCR of RF specificity by co-ligation of complement receptor 2 (CR2). IgG–RF complexes are also pro-inflammatory. TCR, T-cell receptor. Jonathan C. W. Edwards & Geraldine Cambridge; http://www.nature.com/
Humoral Immunity; http://www.cram.com/
The cascade of cytoplasmic factors includes many of those discussed above: PKC, IP3, DAG, calcium, small G proteins, MAP kinases, and transcription factors. Via this sequence of enzymatic events, new mRNA synthesis (note: Jane way incorrectly says gene synthesis in the heading 5-11) occurs as transcription factors bind the DNA. Several anti-rejection drugs, including cyclosporin A and FK506, block T cell activation by selectively interfering with the transcription regulator NFAT.

http://quizlet.com/


The cells of the immune system that make antibodies to invading pathogens such as viruses; They form memory cells that remember the same pathogen for faster antibody production in future infections. http://en.wikipedia.org/
Activation to products- a summary; http://en.wikipedia.org/
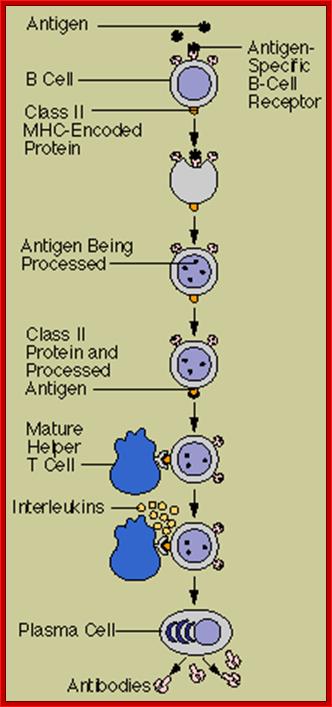
Activation of B cells to make antibodies; http://people.eku.edu/

T cell dependent B cell activation: http://en.wikipedia.org/
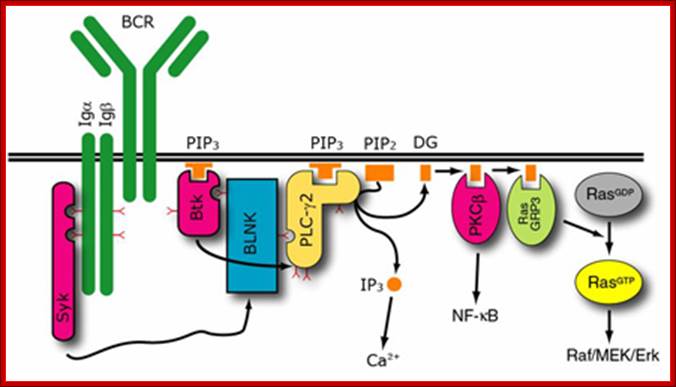
MAPK pathway via SykBCR MapK pathway; http://www.ibi-lorenzini.it/
B cell receptor (BCR) activation results in the sequential activation of protein tyrosine kinases, which results in the formation of a signaling complex and activation of downstream pathways as shown. Whereas SLP76 is recruited to the membrane through GADS and LAT, the mechanism of SLP65 recruitment is unclear. Studies have indicated two mechanisms: (a) direct binding by the SH2 domain of SLP65 to immunoglobulin (Ig) of the BCR complex or (b) membrane recruitment through a leucine zipper in the amino terminus of SLP65 and an unknown binding partner. ADAP, adhesion-and degranulation-promoting adaptor protein; AP1, activator protein 1; BTK, Bruton’s tyrosine kinase; DAG, diacylglycerol; GRB2, growth-factor-receptor-bound protein 2; HPK1, haematopoietic progenitor kinase 1; InsP3, inositol-1,4,5-trisphosphate; ITK, interleukin-2-inducible T-cell kinase; NCK, noncatalytic region of tyrosine kinase; NF-B, nuclear factor B; PKC, protein kinase C; PLC, phospholipase C; PtdIns(4,5)P2, phosphatidylinositol-4,5-bisphosphate; RAS-GRP, RAS guanyl-releasing protein; SOS, son of sevenless homologue; SYK, spleen tyrosine kinase. http://what-when-how.com/
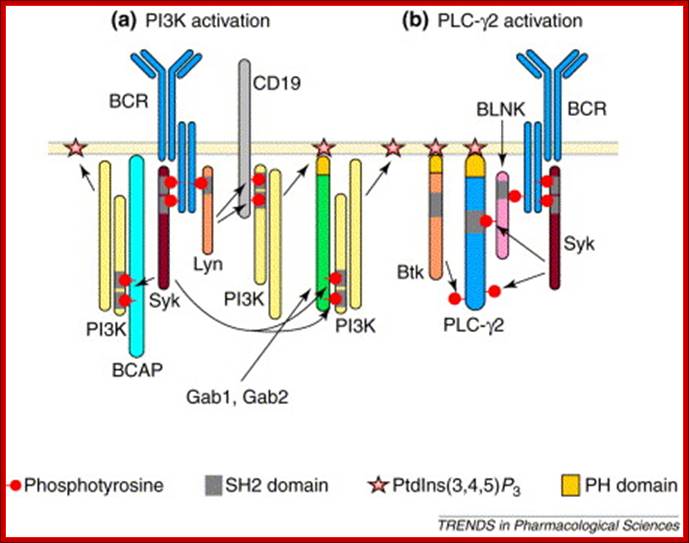
B-cell antigen receptor (BCR)-induced membrane recruitment and activation of phosphatidylinositol 3-kinase (PI3K) and phospholipase C-γ2 (PLC-γ2); (a) Recruitment to the plasma membrane allows PI3K to phosphorylate phosphatidylinositol (4,5)-bisphosphate [PtdIns(4,5)P2] to produce phosphatidylinositol (3,4,5)-trisphosphate [PtdIns(3,4,5)P3]. BCR-induced phosphorylation of the CD19, Gab1, Gab2 and BCAP (B-cell adaptor for phosphoinositide 3-kinase) docking proteins on YxxM motifs mediates the recruitment of PI3K to the plasma membrane. CD19 is a transmembrane protein, whereas Gab1 and Gab2 associate with the membrane via binding of their plekstrin-homology (PH) domains to PtdIns (3, 4, 5)P3. It is not known how BCAP associates with lipid-raft micro domains of the plasma membrane. (b) Binding of the SH2 domain of PLC-γ2 to phosphotyrosine-containing sequences on the BLNK (B-cell linker protein) adaptor protein causes PLC-γ2 to translocate to the plasma membrane. The association between PLC-γ2 and the plasma membrane is stabilized by binding of PtdIns(3,4,5)P3 to the PH domain of PLC-γ2. PLC-γ2 is then activated by phosphorylation by the Syk and Btk tyrosine kinases. Activated PLC-γ2 cleaves PtdIns(4,5)P2 into the second messengers inositol (1,4,5)-trisphosphate [Ins(1,4,5)P3] and diacylglycerol; www.cell.com
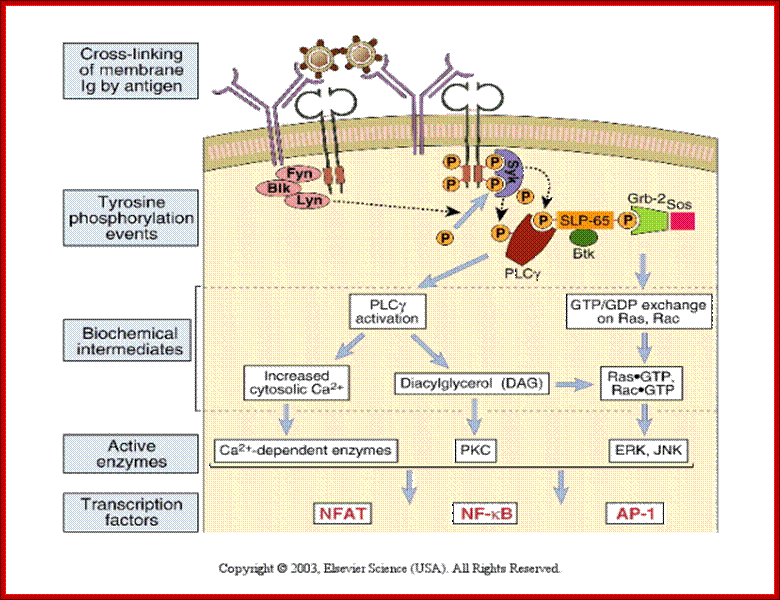
http://slideplayer.com
|
Different pathways · The Ras-MAP kinase pathway is activated in antigen- stimulated B cells. · PLC- is activated in response to BCR signaling and also activates downstream signaling pathways. |
|
|
|
|
|
|
T cell Activation:
T cell maturation in thymus also progresses in stages distinguished by expression of entire receptors’ genes, CD4 and CD8 co receptor molecules and location in thymus. The earliest T cell lineage immigrants to thymus do not express TCRs or CD4 or CD8 molecules. Developing T cells within Thymus (thymocytes) initially populate the outer cortex, where they undergo proliferation, rearrangement of TCR genes and surface expression of TCR CD3, TCR CD4 and CD8. As cells mature they migrate from the cortex to the medulla.
The least mature thymocytes called pro-T cells are Cd4^- and CD8^- double negative and the TCR genes are in germ line configuration. In the pre-T stage, thymocytes remain double negative, but VDJ recombination occurs in TCR beta chain locus. Primary beta chain transcripts are expressed and processed to bring beta segments adjacent to VDJ complex and beta chain polypeptides are produced. The beta chain associates with the invariant pre-T alpha protein to form a pre TCR. The pre TCR transduces signals that inhibit rearrangements on the other beta chain alleles (allelic exclusion) and promote CD4 and CD8 expression and further proliferation of immature thymocytes to the CD4+CD8+ (double positive) stage of T cell development, V-J recombination in the alpha locus , alpha chains are produced and low levels of TCR are expressed on cell surface.
Selection process drive maturation of TCR expressing double positive thymocytes and shape the T cell repertoire towards self MHC restriction and self-tolerance. Positive selection of CD4+CD8+ TCR-alpha/beta thymocytes require low avidity recognition of peptide –MHC complexes on thymic epithelial cells, leading to a rescue of the cells from PCD. Negative selection of CD4/CD8 plus TCR alpha/beta double positive thymocytes occur when these cells recognize with high avidity, antigens that are present at high concentration in thymus. This process is responsible for tolerance to many self-antigens. Most of the cortical thymocytes do not survive this selection process. As the surviving TCR-alpha/beta cells mature they move into the medulla and become CD4, CD8plus or CD4/CD8 minus. Medullary thymocytes acquire the ability to differentiate into either helper or cytotoxic effector cells and finally emigrate to peripheral lymphoid tissues. Some peptides in which TCR contact residues are altered may induce partial T cell responses or inhibit T cell activation by poorly understood biochemical.
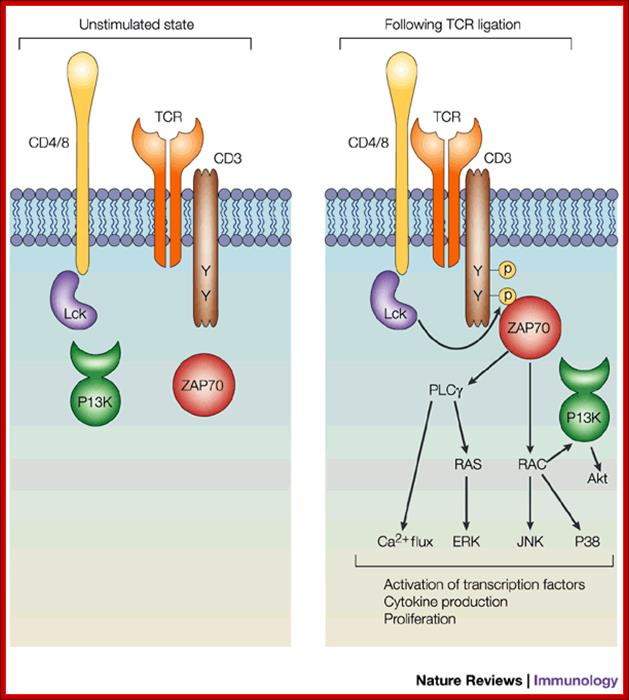
The figure shows the state of unstimulated T cell and stimulated T cell. The binding of antigen stimulates receptors associated signal transducers for cytokine production and proliferation. www.nature.com
.
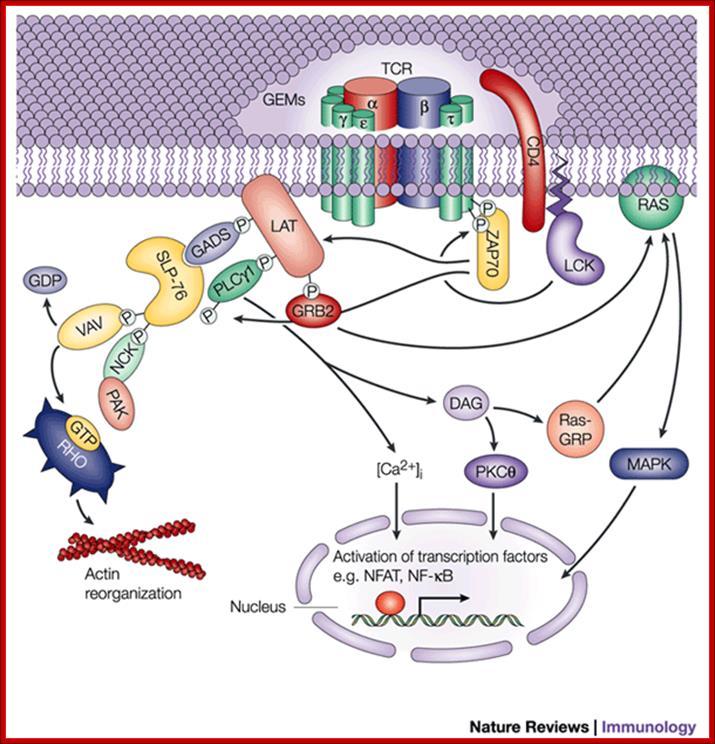
Adaptor proteins, molecules that mediate intermolecular interactions, are now known to be as crucial for lymphocyte activation as are receptors and effectors. Extensive work from numerous laboratories has identified and characterized many of these adaptors, demonstrating their roles as both positive and negative regulators. Studies into the molecular basis for the actions of these molecules shows that they function in various ways, including: recruitment of positive or negative regulators into signalling networks, modulation of effector function by allosteric regulation of enzymatic activity, and by targeting other proteins for degradation. This review will focus on a number of adaptors that are important for lymphocyte function and emphasize the various ways in which these proteins carry out their essential roles. Positive and negative regulation of t-cell activation by adaptor proteins;
Clustering of TCR by antigens recognition triggers intracellular signaling pathways that result in the production of transcription factors which activate a variety of genes in T cells. Intracellular signaling may be divided into membrane events, cytoplasmic signaling pathways and nuclear transcription of genes. Membrane events include the recruitment and activation of protein tyrosine kinases into the TCR complexes; the phosphorylation of TCR complex constituents (e.g. the chi chains) and the recruitment of protein tyrosine kinases, especially ZAP-70 and adapter proteins. Cytoplasmic signaling pathway leads to the activation of effector enzymes, such as kinases ERK, JNK, and PKC and the phosphatase calcineuriun. These enzymes contribute to the activation of transcription factors such as NFAT, AP1 and NFkB which function to enhance gene expression in antigen stimulated T cells.
Some peptides in which TCR contact residues are altered may induce partial T cell responses or inhibit T cell activation by poorly understood biochemical mechanisms.

T cell microarray during development; www.keyword-suggestions.com
Affinity maturation leads to the increased affinity of antibodies during the course of T cell dependent humoral responses. Affinity maturation is a result of somatic hyper mutation of Ig heavy and light chain genes followed by selective survival of the B cells that produce the high affinity Abs and bind antigens displayed by FDCs in the germinal centers.
Some of the progeny of germinal center B cells differentiate into antibody secreting cells that migrate to extracellular regions of secondary lymphoid organs and BM. Other progeny become memory cells that live for a long periods, recirculate between lymph nodes and spleen and respond rapidly to subsequent exposure to antigens for which they are already stimulated, this is because of antigen stimulated high affinity antibody secretors.
Thymus independent (YI) antigens are non-protein antigens that induce humoral immune responses without the involvement of helper T cells. Many TI antigens, including polysaccharides, glycolipids and nucleic acids, polyvalent, can be cross linked multiple membrane Ig molecules on B cells and activate complement, thereby activating B cells without the help of T cells. TI antigens stimulate antibody responses in which there is limited or no heavy chain class switching, affinity maturation, or memory B cells generation. Because these feature are dependent on helper T cells, which are not activated by non-protein antigens.
Antibody feedback is a mechanism by which humoral immune responses are down regulated when enough antibodies have been produced and soluble antibody-antigen complexes are present. B-cell membrane Ig and B cell receptor for Fc portions of IgG. Called Fc gamma RIIB, are clustered together by antibody-antigen complexes. This activates an inhibitory signaling cascade through the cytoplasmic tail of FcyRII that terminates the activation of B cell.
Signal Transduction in T Cell Activation and Anergy (Tolerance): Life and Medical Sciences (LIMES) Institute, University of Bon:
Authors work characterizes biochemical processes that integrate signals from the antigen receptor and from co-stimulatory/co-inhibitory molecules. The mechanistic cooperation of the T cell antigen receptor and CD28, which is required for productive T cell activation, is not very well understood. Furthermore, the mode of action of negative regulatory receptors, such as PD-1 or CTLA-4, which induce anergy/tolerance or contribute to the attenuation of an inflammatory response, needs to be linked to intracellular signaling.
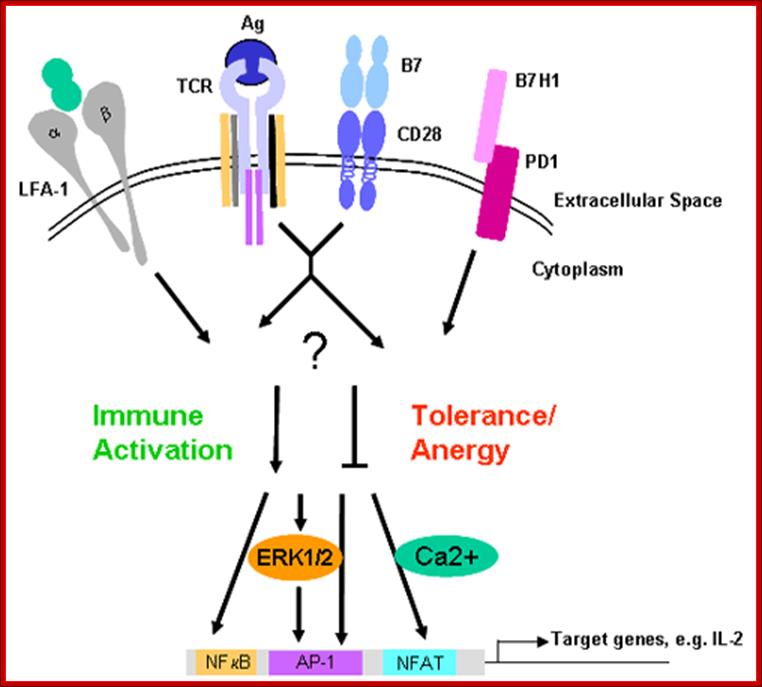
Here authors have analyzed the role of the cytohesin family of proteins. These are guanine-nucleotide exchange factors, which also play a major role in the control of cell adhesion and migration. Furthermore, we have recently identified novel proteins that appear to be centrally involved in the control of T cell activation. In the course of this project, we collaborate with P. Knolle (IMMEI, Bonn, SFB704), who provides a murine in vivo tolerance model. Major branches of T cell activation (Ca2+/NFAT, Ras/MAPK, NFkB) are investigated by biochemical or functional assay systems. In the recent past we have also begun to put a strong emphasis on live visualization of cellular processes using high-end microscopical techniques. www.keywordsking.com
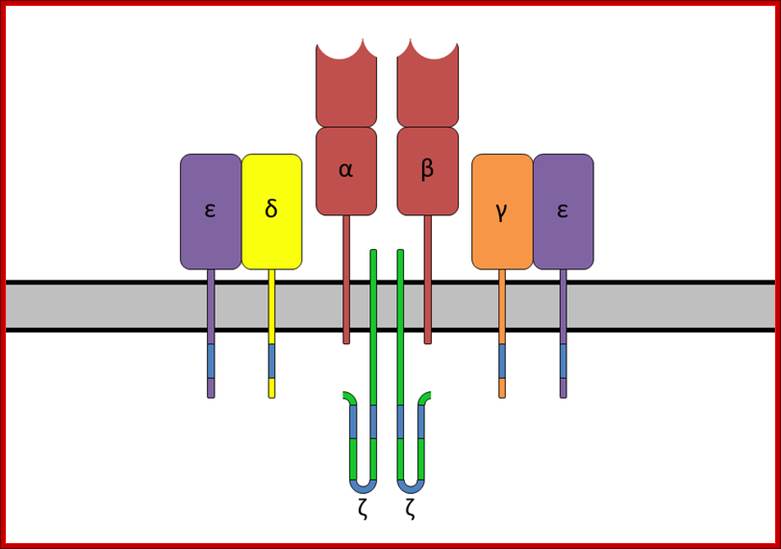
The T-cell receptor complex with TCR-α and TCR-β chains, CD3 and ζ-chain accessory molecules; ITAMs are represented in blue on the tails of the CD3 subunits. https://www.boundless.com
ITAMs are important for signal transduction in immune cells. Hence, they are found in the tails of important cell signaling molecules such as the CD3 and ζ-chains of the T cell receptor complex,[1] the CD79-alpha and -beta chains of the B cell receptor complex, and certain Fc receptors. The tyrosine residues within these motifs become phosphorylated following interaction of the receptor molecules with their ligands and form docking sites for other proteins involved in the signaling pathways of the cell.
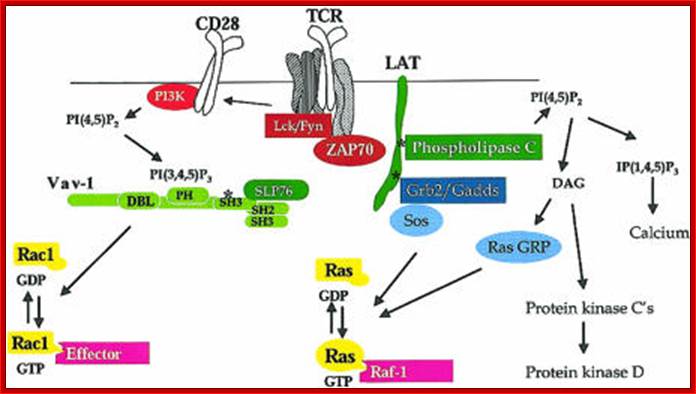
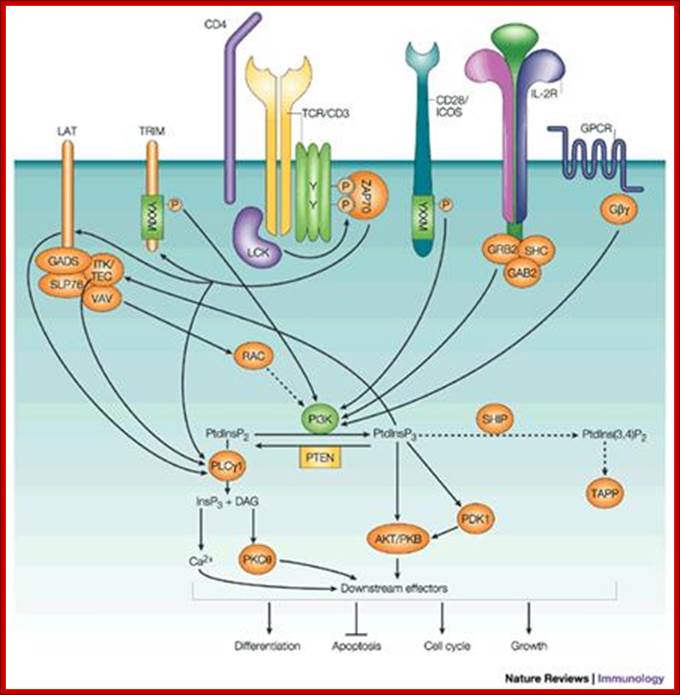
Inositol lipid metabolism in T cells is little different from that of BCR signaling. The combined action of triggered antigen receptors and costimulatory molecules activates cytosolic tyrosine kinases which link via adaptors to effector enzymes PLCγ1 and PI3K. These two enzymes act on the same substrate PtdIns (4,5)P2. PLCγ1 hydrolyses PtdIns(4,5)P2 to produce inositol triphosphate and diacylglycerol, whereas PI3Ks phosphorylates PtdIns(4,5)P2 to produce PtdIns(3,4,5)P3. Inositol polyphosphates, DAG and PtdIns(3,4,5)P3 then initiate key intracellular signaling molecules that transduce signals away from the T-cell membrane into the cell interior. The major serine kinases involved in T-cell activation are shown and their intracellular location, when activated, is indicated. M* indicates the kinase is active at the plasma membrane; C* indicates the kinase is active in the cytosol; N* indicates the kinase is active in the nucleus
GTPase regulation in T cells; GTPases cycle between an active GDP-bound state and an active GTP-bound state. When GTP-bound, GTPases can bind to their effectors. The pathways involved in the regulation of GTPases Rac-1 and Ras are shown. The key steps are thought to be activation of guanine nucleotide exchange proteins: Vav-1 for Rac-1 and SOS and Ras-GRP for Ras. Other GTPases important for T-cell activation are RhoA and CDc42, but the biochemical steps in their activation are not known. The main effector for Ras is the serine kinase Raf-1. Rac-1 is a potent regulator of the actin cytoskeleton and NFκB. Many Rac-1 effectors have been described in other cell types, but Rac-1 effectors in T cells are not known
Antibodies produced by a mature plasma B cell
recognize the same specific antigen epitope as the BCR on the cell's surface.
This is because the BCR on the plasma cell is actually a surface membrane bound
form of the antibody that is made by that cell. Antibodies are also known as immunoglobulins
and gamma-globulins. They are typically Y-shaped molecules
formed from paired heavy and light polypeptide chains. The arms of antibody
molecules end in a variable region that, similar to the TCR and BCR, combines
with a specific antigen epitope in a lock-and-key manner. http://sphweb.bumc.bu.edu/
produced by a mature plasma B cell
recognize the same specific antigen epitope as the BCR on the cell's surface.
This is because the BCR on the plasma cell is actually a surface membrane bound
form of the antibody that is made by that cell. Antibodies are also known as immunoglobulins
and gamma-globulins. They are typically Y-shaped molecules
formed from paired heavy and light polypeptide chains. The arms of antibody
molecules end in a variable region that, similar to the TCR and BCR, combines
with a specific antigen epitope in a lock-and-key manner. http://sphweb.bumc.bu.edu/
IP 3 R functions in cells of the immune system:
Focus Article
Askar M. Akimzhanov, Darren Boehning;
Calcium ions regulate almost all cellular processes. Inositol 1,4,5‐trisphosphate receptors (IP3Rs) are ubiquitous calcium channels which mediate calcium release primarily from endoplasmic reticulum stores. IP3Rs are activated by the soluble second messenger inositol 1,4,5‐trisphosphate (IP3), which is produced in response to various stimuli by the action of phospholipase C enzymes. Very soon after the discovery that IP3 is a calcium mobilizing second messenger, it was found that T‐cell receptor signaling is dependent upon IP3R activity. It is now known that cell signaling throughout the immune system is dependent upon IP3R‐dependent calcium release for many critical processes, including cellular activation, proliferation, and death. This channel plays a critical role in orchestrating the immune response in health and disease and thus offers potential as a therapeutic target in immune‐related disorders. WIREs Membr Transp Signal 2012,1:329–339. doi: 10.1002/wmts.27
Clonally distributed TCRs on T cells recognize peptide–MHCs on APC cells. The APCs can be dendrite cells, macrophages, many white blood cells and even B cells. Responses require specific antigen recognition. These TCRs are composed of two disulfide linked polypeptide chains called alpha and beta, they are homologous to Ig’s heavy and light chains. Each of the chains of alpha and beta TCR consists of a V-region and a C-region.
APC consisting of MHCs with an antigenic piece supported by co stimulators such as CD80/86 and B7 proteins. The TCR contains Alpha and Beta components of receptors and added stimulators such as CD4/8 and CD28. The TCRs have antigenic binding complementary regions I their proteins, so a specific TCR binds to to specific antigen which has chosen by the MHC and presented for action.
The V segment of each chain contains three hyper variable complementary determining regions, which form the portion of the receptor that recognizes complex of processed peptide antigens presented by MHC molecules. During T cells’ antigen recognition, the TCR makes contact with a. a residues of the peptide as well as the polymorphic residues of the presenting self MHC accounting for dual recognition of peptides and self molecules.
Askar M. Akimzhanov, Darren Boehning; WIREs Membr Transp Signal 2012
The gamma and delta protein complex of TCR is another clonally distributed heterodimers that are expressed on small subset of alpha and beta negative T cells. These gamma/delta T cells are not MHC restricted and they recognize different forms of antigens than a/b T cells do, including lipids and some small molecules presented by nonpolymorphic MHC- like molecules.
The a/b and d/g heterodimeres are noncovalently associated with four invariantly found on membrane surfaces; three of which are components CD3 and the fourth is the chi chain; The assembly of TCR , CD3 and chi chain is called the TCR complex. When a/b of TCR binds peptide-MHC complex, the CD3 and chi proteins act as transducers and sending signals that initiate the process of T cell activation.
CD4 and CD8 are co receptors expressed on mutually exclusive subsets of mature T cells that bind non polymorphic regions of class II and I MHC respectively. CD4 is expressed on class II–restricted helper cells, CD8 is expressed on class I restricted CTLs. When T cells recognize peptides-MHC complexes, the CD4 and CD8 deliver signals that is critical for initiating T cell responses called signal-2 that are required; this is in addition to TCR complex generated signals ( signal 1). For full T cell activation. A second receptor for B7 called CTLA-4 is induced after T cell activation and function to inhibit responses.
Cd28 is a receptor on T ells that binds to B7 co stimulatory molecules expressed on professional APC CD28 delivers signals, often called signal 2, that are require in addition to TCR cell complex generated signals for full T cell activation. A second receptor for B7 called CTLA4 is induced after T cells activation and functional to inhibit responses.
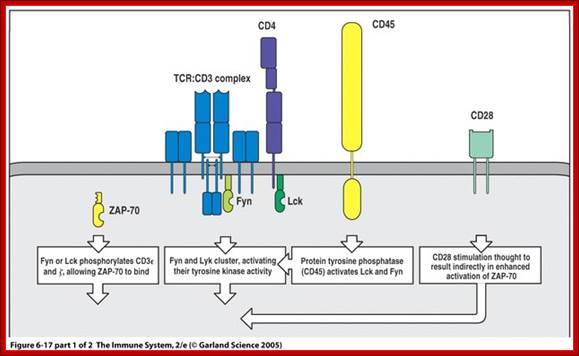
T cell receptors and their associated proteins; www.keywordsuggests.com
The cell integrins LFA1 and VLA4 bind ICAMs and VCAMs are found on the surfaces of other cells. The adhesive interactions are important for stable conjugation of T cells and APCs for T cell migration from blood into tissues. The binding of integrins to their ligands is regulated by changes in the avidity and the expression of integrins and by changes in the expression of their ligands
CD40 ligands and Fas ligands are T cell surface molecules whose expression is induced by T cell activation and that perform specialized effector functions. These adhesive interactions are important for stable conjugation of T cell and APC and for T cell migration from blood into tissues.
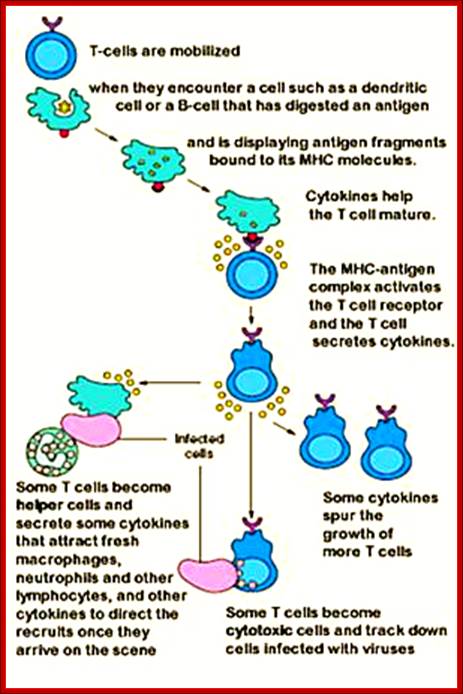
T-cell mobilization, activation and maturation. https://www.pinterest.com
SUBJECT
TOPIC
T cell responses to the antigen and co stimulators include synthesis of cytokines and effector molecules, cellular proliferation, differentiation into effector and memory cells and performance of effector functions. http://kt.h0ok.com
THE
Activation of Cytotoxic T Cells
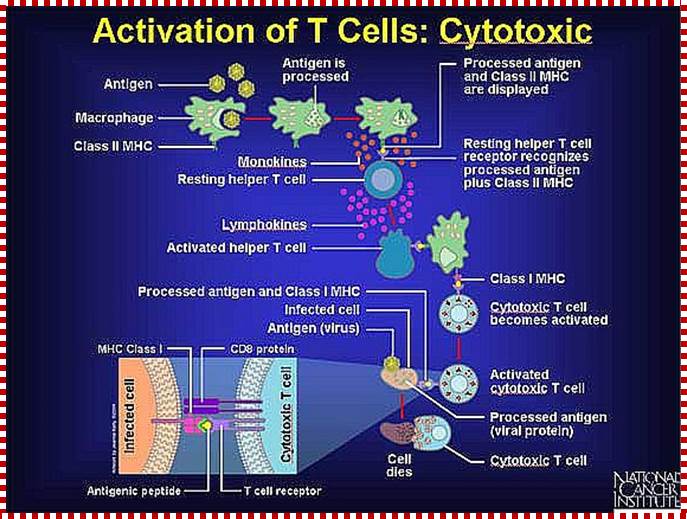
Killer T cells only recognize antigen in the grasp of Class I MHC markers. Here a resting cytotoxic T cell recognizes virus fragments, which are displayed by a macrophage in combination with a Class I MHC marker. A receptor on a circulating, resting cytotoxic T cell (and CD8 protein) recognizes the antigen-protein complex and binds to it. The binding process and an activated helper T cell activate the cytotoxic T cell. Because the surfaces of other infected cells bear the same virus fragments in combination with Class I MHC markers, the activated cytotoxic T cells can quickly recognize, attack, and destroy the diseased cell. http://www.web-books.com
Activation of T helper cells:
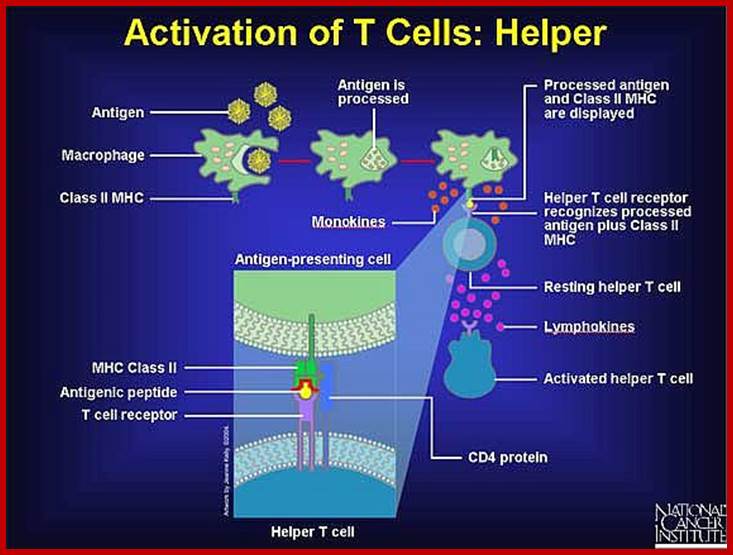
Helper T cells only recognize antigen in the grasp of Class II MHC markers. An antigen-presenting cell--such as a macrophage or a dendritic cell--breaks down the antigen it devours, and then it places small pieces (peptides) on its surface along with a Class II MHC marker. By exhibiting its catch in this way, antigen-presenting cells enable specific receptors on helper T cells to bind the antigen and confirm (via CD4 protein) that an invasion has occurred. http://www.web-books.com
After binding, a resting helper T cell quickly becomes an activated helper T. It assumes command of the immune response, giving orders to increase the number of specific antibody-producing plasma cells and the cytotoxic killer cells needed to quell the attack.
T cell activation by an antigen-presenting dendritic cell; Antigen presenting cells use class II MHC molecules in contrast to other cells. False-color TEM of killer T-lymphocyte; Credit: Edelmann/Science Photolibrary; www.medscape.org
Caption: False-color transmission electron micrograph (TEM) of a single, activated cytotoxic ("killer") T-lymphocyte (left, a class of white blood cell) after delivering the "kiss of death" to its target cell. T-lymphocytes are involved in cell-mediated response to invasion of body by foreign microorganisms, notably viruses and some bacteria, which are protected from antibodies (secreted by B-lymphocytes), by their host cells. "Killer" T- cells recognize antigens on their target cell & bind to the surface membrane. Dark vesicles between 2 cells represent T-cell enzymes involved in the destruction of the target cell membrane

Activated dendritic cells (DCs) express markers that can bind to and activate naïve T cells. When activated, the T cells proliferate to mount an immune response against a targeted antigen; http://www.nature.com
Antigen-presenting cells ingest a microbe, partially degrade it, and export fragments of the microbe—i.e., antigens—to the cell surface, where they are presented in association with class II MHC molecules. A receptor on the surface of the helper T cell then binds to the MHC-antigen complex. But this event alone does not activate the helper T cell. Another signal is required, and it is provided in one of two ways: either through stimulation by a cytokine or through a costimulatory reaction between the signaling proteins, B7, found on the surface of the antigen-presenting cell, and the receptor protein, CD28, on the surface of the helper T cell. If the first signal and one of the second signals are received, the helper T cell becomes activated to proliferate and to stimulate the appropriate immune cell. If only the first signal is received, the T cell may be rendered anergic—that is, unable to respond to antigen.
Dendritic cell
From Wikipedia, the free encyclopedia
Jump to: navigation, search;

Dendritic cells: emerging pharmacological targets of immunosuppressive drugs; Holger Hackstein & Angus W. Thomson; http://www.nature.com
Dendritic cells (DCs) are immune cells forming part of the mammalian immune system. Their main function is to process antigen material and present it on the surface to other cells of the immune system. That is, dendritic cells function as antigen-presenting cells. They act as messengers between the innate and adaptive immunity.
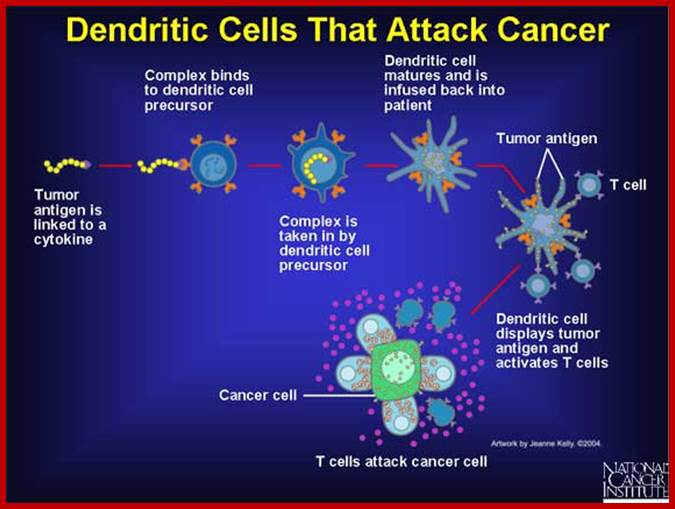
Figure; The above figure shows a tumor antigen linked to a cytokine bind to a dendritic cell. The dendritic cell processes the antigen and presents it to cytotoxic T cells. The activated cytotoxic T cells now recognize the cancer cell and destroy it. http://www.web-books.com
What is the difference between Killer Cells and Natural Killer Cells?
I think one might be confusing
killer cells with natural killer cells (NK cells). Cytotoxic T-cells are the
same as killer T-cells and are part of the induced immune system. NK cells are
part of the innate system and circulate constantly (Babybel56). CD8+ cells are mostly meant to
fight intracellular parasites (usually viruses) and cancer cells, and thus
recognize foreign antigens that are produced by the cells themselves. Thus,
virtually any cell in the body is an APC for them. If a CD8+ cell recognizes a
foreign antigen on a cell's surface, it kills this cells - hence the 'killer
cell' name (this is to prevent viral infection or cancer from spreading). The
"killing" happens usually by forcing the target cell undergo
apoptosis. Almost all cells in the body have MHC I molecules on their surface
and CD8+ cells recognize antigens only when they are presented on MHC I. NK
cells, to the contrary, do not require APCs and they do not adapt according to
the type of antigen or infection (unlike T and B cells, who undergo clonal
expansion and increased antibody affinity etc.). Thus, NK cells are considered
to be a part of the innate immune system: they always recognize same, common
patterns on microbial.
There are many subtypes of all immune cells, including CD4+ and CD8+ cells as
well as NK cells. Not all of the subtypes are even known yet. However, the key
features are quite well known, and even though they might seem complex, there
is at least neat logic behind all of it.
Best Answer - Chosen by Asker:
Natural Killer Cells (NK Cells) work in conjunction with the innate immune response. Large granular lymphocytes; non-phagocytic; mainly target malignant, foreign transplanted, and viral-infected cells as well as cells marked for destruction; spends its life looking for MHC-1 cells. If MHC-1 is found, it will ignore it. If it is not found, then the cell is destroyed. Destruction depends on the balance between "kill" and "don't kill" signals. Secrete interferon (IFN-gamma) which activates macrophages. DO NOT HAVE TCR RECEPTORS! They were named "natural killers" because of the initial notion that they do not require activation in order to kill cells that are missing "self" markers known as MHC-1. Killer T Cells (aka: Cytotoxic T Lymphocytes-- (CTL)) work in conjunction with the adaptive immune response and work in a similar way to the NK cells.
They
have T Cell receptors (TCR) that recognize MHC-1.
Both display CD8 markers. Both kill by either inducing apoptosis through activation
of the Fas ligand on cells OR by injecting a granzyme "bomb" into the
"bad cells. Neutrophils are totally different from both. They are
phagocytic (cell-eating) granulocytes that help to make up a portion of the
cellular component of the innate immune system. Both the Innate and Adaptive
immune system have a cellular response (component) as well as a humoral
response (component):
Innate Cellular response is made up of macrophages, neutrophils, Basophils, Eosinophils,
and NK cells. (They are the CELLS that respond).
Innate Humoral response is made up of Complement which serves many different
functions. (It is NO A CELLULAR component)
Activation NK cells: Jean-Saville Cummings, PhD, Vincent Arnold and Celine Didier;
From Wikipedia, the free encyclopedia
Jump to: navigation, search
Natural killer cells (or NK cells) are a type of cytotoxic lymphocyte critical to the innate immune system. The role NK cells play is analogous to that of cytotoxic T cells in the vertebrate adaptive immune response. NK cells provide rapid responses to virally infected cells and respond to tumor formation, acting at around 3 days after infection. Typically immune cells detect MHC presented on infected cell surfaces, triggering cytokine release causing lysis or apoptosis. NK cells are unique, however, as they have the ability to recognize stressed cells in the absence of antibodies and MHC, allowing for a much faster immune reaction. They were named “natural killers” because of the initial notion that they do not require activation in order to kill cells that are missing “self” markers of major histocompatibility complex (MHC) class 1. NK cells are defined as large granular lymphocytes (LGL) and constitute the third kind of cells differentiated from the common lymphoid progenitor.
NK cells are known to differentiate and mature in the bone marrow, lymph node, spleen, tonsils and thymus where they then enter into the circulation. [3]. NK cells differ from Natural Killer T cells (NKT) phenotypically, by origin and by respective effector functions; often NKT cell activity promotes NK cell activity by secreting IFNγ. In contrast to NKT cells, NK cells do not express T-cell antigen receptors (TCR) or Pan T marker CD3 or surface Immunoglobulins (Ig) B cell receptors, but they usually express the surface markers CD16 (FcγRIII) and CD56 in humans, NK1.1 or NK1.2 in C57BL/6 mice. Up to 80% of human NK cells also express CD8.
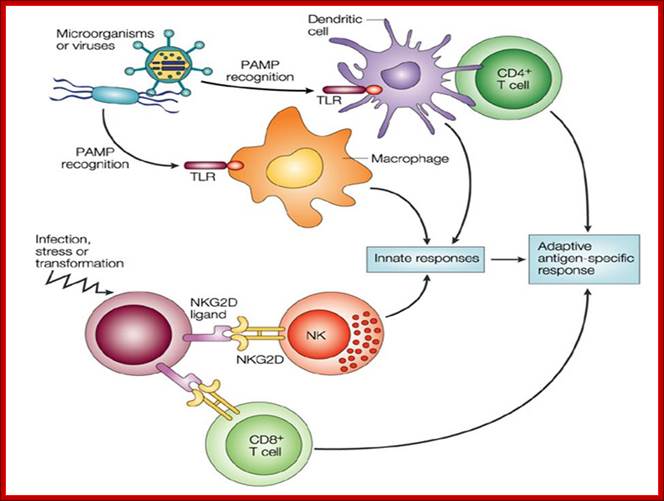
Reciprocal activation of NK cells and DCs through NK/DC interactions is now recognized to be an important dynamic issue connecting the innate and adaptive arms of the immune system. An activation of NK cells was reported in response to DC-based vaccination, suggesting that NK cell activation play a role in generating an immune response to vaccines. Additional evidence is still required to elucidate the significance of human NK/DC cross-talk in vivo and to identify the best correlates of protection against infection or disease progression. Therefore, our aim is to evaluate how DC loaded with different kind of HIV antigens (lipopeptides and MVA constructions used in ANRS trials) modulates the NK/DC cross-talk in vitro in order to mount an anti-HIV protective response. We plan to characterize NK cells activated by HIV-loaded DC in term of receptor expression, cytolytic and secretory capacities. We used an in vitro co-culture model of NK cells with HIV-loaded monocyte-derived DC (MDDC) or not in autologous condition. We expect to define the best combination of HIV antigens able to activate the appropriate NK cell subset to help the anti-HIV T cell specific response. https://humanphisiology.wikispaces.com; https://www.pinterest.com
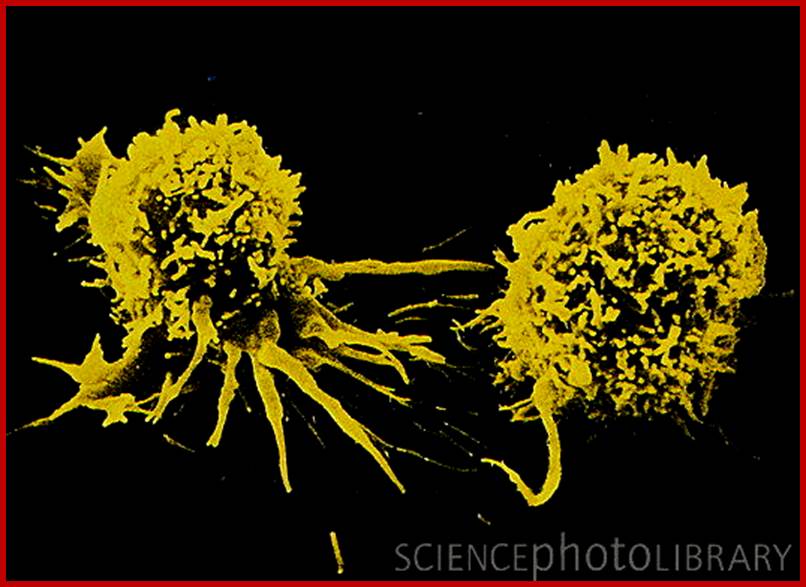
NK cells- Caption: Cancer immunotherapy: false-color scanning electron micrograph (SEM) of two lymphokine-activated killer (LAK) cells. Upon activation by Interleukin-2 (IL-2) natural killer (NK) cells have displayed anti-cancer activity in laboratory conditions. IL-2 is released naturally by the immune system following activation by cancer cells. In LAK immunotherapy, a patient's white blood cells are removed & cultured with IL-2 to allow LAK cells to develop. The product, when reintroduced into the patient, has resulted in a significant reduction in tumour size in some patients with certain types of cancer. Mag: x6700 at 5x7inch, x2800 at 6x7cm size. www.sciencephoto.com
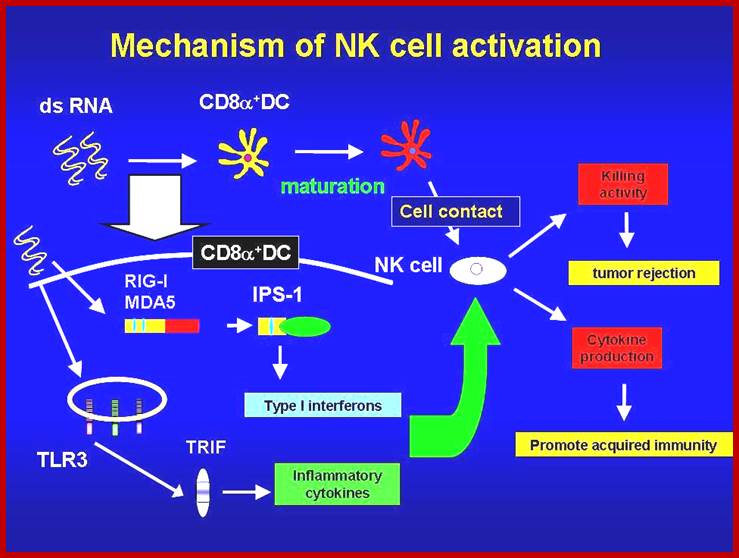
NK cells play essential roles in eliminating virally infected cells and tumor cells. Polyriboinosinic-polyribocytidylic acid (poly I:C), a double-stranded RNA analogue recognized by MDA5 and TLR3, activates NK cells in vivo. MDA5 and TLR3 signal through distinct adaptor molecules, IPS-1 and TRIF, respectively. However, it remains unclear how NK cells are activated by poly I:C in vivo. Here, we demonstrate that the IPS-1-dependent and the TRIF-dependent pathways are essential for NK cell activation to poly I:C stimulation in mice, whereas deficiency in either IPS-1 or TRIF only modestly impairs the poly I:C-induced NK cell activation. Furthermore, both IPS-1 and TRIF contributed to suppression of implanted B16 tumor growth in response to poly I:C administration via NK cell activation. Presence of IPS-1 and TRIF in dendritic cells (DCs), but not NK cells, was required for production of IFN-γ to poly I:C in NK cells in vitro. Moreover CD8α+ conventional dendritic cells (cDCs), but not CD8α- cDCs, expressed genes for type I IFNs, IL-6 and IL-12p40 in response to poly I:C stimulation, and were also responsible for inducing IFN-γ production in NK cells. Taken together, poly I:C activates the IPS-1- and TRIF-dependent pathways in CD8α+ cDCs, which in turn leads to NK cell activation. http://hostdefense.ifrec.osaka-u.ac.jp