Immunology 4
Antigen presentation And MHCells:
Antigen Processing and Presentation:
Pathogens in form of bacteria, viruses and other parasites enter our body from different roots. Many of them are inactivated and killed by innate immune mechanism. But a large number of them escape from innate system. Many of them infect cells and there they multiply. But our body cells are capable of fighting against such pathogens. Pathogens have their surface antigens such as proteins, glycoproteins, surface glycolipid etc, which act as antigens. Our body is endowed with cellular system which can fight against such invaded pathogens. Fighting against pathogens is performed by an ingenious cellular system called Immune system. There are many cellular systems that fight pathogens. One of the cell systems is B cells that produce antibodies and the second is T cells that seek for pathogenic molecules and search such cells that have pathogens and destroy. To find out what cells have such pathogens, there is another cellular system that processes the pathogen associated proteins or such components and presents on the surface of the infected cells. They are Antigen presenting cells (APCs) where they express major histocompatibility complexes (MHCs) which present the pathogen antigens on the external surface. Most of the cells in the body are capable of presenting antigens on their surface using MHC class of docking proteins. Among them Dendrite and Macrophages are efficient APCs. The T cells recognize antigens bund by MHCs. CD4 helper T lymphocytes recognize antigens associated with class II MHC gene products. Class II MHC has restricted recognition. Cells with CD8 CTLs recognize antigens in association with MHC class I complex.
NK cells at the interface between innate and adaptive immunity.

Proteins complex assembly among the proteins synthesized in cytosol- Hierarchial presentarion. Moretta A, Marcenaro E, Parolini S, Ferlazzo G, and Moretta L.; www.yeastrc.org
http://biosiva.50webs.org/
In recent years a novel concept has emerged indicating that the actual role of natural killer (NK) cells is not confined to the destruction of virus-infected cells or tumors. Indeed, different NK subsets exist that display major functional differences in their cytolytic activity, cytokine production and homing capabilities
Early liaisons between cells of the innate immune system in inflamed peripheral tissues, where crosstalk between natural killer (NK) cells and myeloid dendritic cells (DCs) takes place which results in NK-cell activation and DC maturation. Activated NK cells acquire the ability to kill DCs that have failed to undergo complete maturation ('DC editing'). Recent studies have revealed that this crosstalk can be promoted by pathogen-derived products that activate different innate immune cell types directly and simultaneously through their Toll-like receptors (TLRs).
Toll-Like Receptors: The Interface between Innate and Adaptive Immunity; Peter G. Tipping
Innate and adaptive immunity have traditionally has been considered as largely separate though complimentary mechanisms of defense against microbial threats. The adaptive system, being evolutionally newer and having the capacity for selectivity, adaptation, amplification, and memory, has arguably been regarded as more sophisticated and potent. However, recent advances in our understanding of the nature and functions of Toll-like receptors (TLR) has sparked a new appreciation of the extensive interdependence and cross-talk between innate and adaptive responses and has led to renewed interest and higher regard for the contribution of the innate arm of the immune network
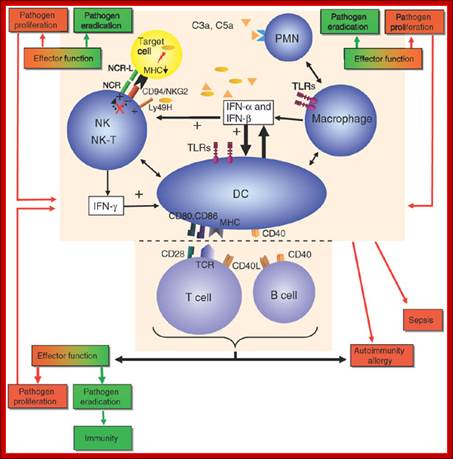
Figure. The interface between innate and adaptive immunity and the consequences
of success or failure. Kasper Hoebe, Edith Janssen & Bruce Beutler; http://www.nature.com/
Essential to the successful removal of pathogens is the early recognition of
microbes by components of the innate immune system. These involve the
complement system, specialized receptors expressed on NK cells and the family
of TLRs that are expressed on myeloid as well as lymphoid cells and that
recognize specific microbially derived molecular structures. Successful
engagement of some of these pathways leads to an inflammatory response with
destruction of the pathogen alongside the enlistment of DC and T cell and/or B
cell interactions. A well-orchestrated innate and adaptive immune response will
lead to pathogen eradication and host immunity (green). Failure to efficiently
discriminate self from non-self in innate as well as adaptive immunity can lead
to pathogen proliferation and ultimately sepsis (red) and may also be the cause
for development and maintenance of autoimmune diseases and allergy (red).
CD40L, CD40 ligand; IFN, interferon; MHC, major histocompatibility complex;
PMN, polymorphonuclear cells; TCR, T cell receptor; NCR, natural cytotoxicity
receptor; NCR-L, natural cytotoxicity receptor−ligand. Kasper Hoebe, Edith Janssen & Bruce
Beutler
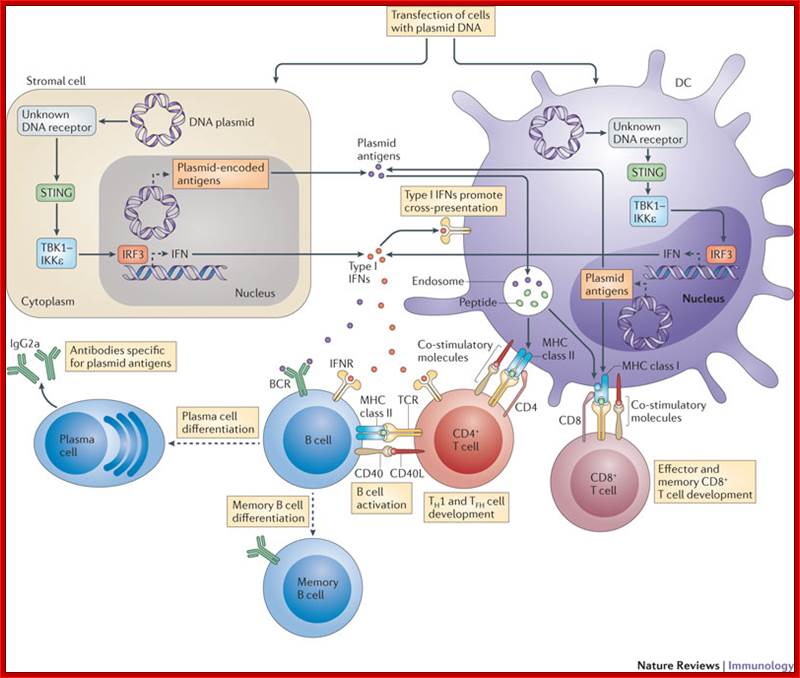
Mechanism of DNA vaccination: Nucleic acid sensing at the interface between innate and adaptive immunity in vaccination; Christophe J. Desmet & Ken J. Ishii; The plasmid DNA used in DNA vaccination may directly transfect stromal cells (such as muscle cells) or dendritic cells (DCs). In these cells, a cytosolic DNA receptor that has not yet been identified induces the activation of TANK-binding kinase 1 (TBK1) and IκB kinase-ε (IKKε) through stimulator of IFN genes (STING), leading to the activation of interferon-regulatory factor 3 (IRF3) and resulting in the production of type I interferons (IFNs). The antigens encoded by the transfected plasmid DNA can also be expressed in stromal cells and DCs. In DCs, these antigens may be directly processed and presented on MHC class I molecules to naive CD8+ T cells. Alternatively, antigens may be indirectly acquired by DCs from stromal cells and then cross-presented to CD8+ T cells or presented to naive CD4+ T cells on MHC class II molecules. Type I IFN expression by stromal cells and DCs seems to be important for promoting the cross-presentation activity of DCs, as well as for the differentiation of T helper 1 (TH1) cells and the promotion of TH1-type isotype switching in B cells. BCR, B cell receptor; CD40L, CD40 ligand; TCR, T cell receptor; TFH, T follicular helper. http://www.nature.com/
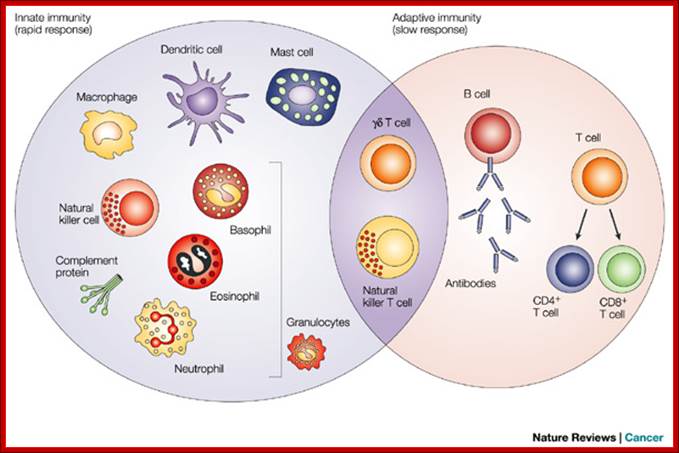
The innate immune response functions as the first line of defence against infection. It consists of soluble factors, such as complement proteins, and diverse cellular components including granulocytes (basophils, eosinophils and neutrophils), mast cells, macrophages, dendritic cells and natural killer cells. The adaptive immune response is slower to develop, but manifests as increased antigenic specificity and memory. It consists of antibodies, B cells, and CD4+ and CD8+ T lymphocytes. Natural killer T cells and gamma-delta T cells are cytotoxic lymphocytes that straddle the interface of innate and adaptive immunity (Dranoff 2004). Dendritic cells are leucocytes that get their name from their surface projections that resemble the dendrites of neurons. They are found in most tissues of the body and are particularly abundant in those that are interfaces between the external and internal environments, e.g., skin and the lining of the gastrointestinal tract. Dencritic cells present antigen/self-antigen complexes that activate T-cells. Gamma-delta cells are primarily found in the intestine, the lining of the vagina, and the skin. They encounter antigens at those locations and, therefore, serve as a first line of defence.;http://people.eku.edu/
The innate immune response functions as the first line of defense against infection. It consists of soluble factors, such as complement proteins, and diverse cellular components including granulocytes (basophils, eosinophils and neutrophils), mast cells, macrophages, dendritic cells and natural killer cells. The adaptive immune response is slower to develop, but manifests as increased antigenic specificity and memory. It consists of antibodies, B cells, and CD4+ and CD8+ T lymphocytes. Natural killer T cells and gamma-delta T cells are cytotoxic lymphocytes that straddle the interface of innate and adaptive immunity (Dranoff 2004). Dendritic cells are leucocytes that get their name from their surface projections that resemble the dendrites of neurons. They are found in most tissues of the body and are particularly abundant in those that are interfaces between the external and internal environments, e.g., skin and the lining of the gastrointestinal tract. Dencritic cells present antigen/self-antigen complexes that activate T-cells. Gamma-delta cells are primarily found in the intestine, the lining of the vagina, and the skin. They encounter antigens at those locations and, therefore, serve as a first line of defense. These DC specific Aggresome Like Induced Structures (DALIS) are transient. The majority of the proteins forming DALIS are newly synthesized DRiPs, which are poorly degraded. DRiPs storage into DALIS is strongly correlated with the inhibition of the autophagic flux. We authors mentioned have demonstrated (Head of Project Dr. Evelina Gatti ) that ubiquitination of the cytoplasmic tail of HLA-DR ß-chain is key for the accumulation and degradation of peptide-loaded MHC II in the lysosomes of immature in human monocyte-derived DCs and this ubiquitination ceases upon DC maturation.
DALIS (ubiquitin in red) in maturing DCs (MHC II in green).
Copyright P
Pierre, CIML; http://www.ciml.univ-mrs.fr/
Macrophages are derived from white blood cells and are found in the tissues. Their role is to clear cellular debris and pathogens from the body through phagocytosis. In normal cellular immune system functioning, the macrophage ingests a parasite into a phagosome (Figure 8), which fuses to one of the cell's many late endosomes and lysosomes. Parasite proteins are then degraded into short peptide fragments, which the macrophage then presents in the context of MHC class II molecules to CD4+ helper T cells, another type of white blood cell, in the lymph node. Macrophages may also display parasite peptides in the context of MHC class I molecules to CD8+ cytotoxic T cells. This activates the cytotoxic T cells to proliferate and destroy the infected macrophage through the secretion of the cytokine interferon gamma (IFN-g) (7).; Macrophages have multifaceted role, first they take in pathogens and process them into small antigenic molecules and the same are loaded on to MHC cells and loaded on to cell surface membranes. http://web.stanford.edu/
Macrophages act as phagocytes among many functions One important role of the macrophage is the removal of necrotic cellular debris in the lungs. Removing dead cell material is important in chronic inflammation, as the early stages of inflammation are dominated by neutrophil granulocytes, which are ingested by macrophages if they come of age.
The removal of necrotic tissue is, to a greater extent, handled by ''fixed macrophages'', which will stay at strategic locations such as the lungs, liver, neural tissue, bone, spleen and connective tissue, ingesting foreign materials such as pathogens, recruiting additional macrophages if needed.
When a macrophage ingests a pathogen, the pathogen becomes trapped in a phagosome, which then fuses with a lysosome. Within the phagolysosome, enzymes and toxic peroxides digest the pathogen. However, some bacteria, such as ''Mycobacterium tuberculosis'', have become resistant to these methods of digestion. Macrophages can digest more than 100 bacteria before they finally die due to their own digestive compounds.
Macrophages can be used to generate Hybridomas to generate monoclonal antibodies. The generation of macrophage-hybridomas, obtained by somatic cell fusion between macrophage-enriched C3H.eB spleen cell population and the drug resistance MPC-II myeloma cell lines.
http://www.slideplayer.com.br/
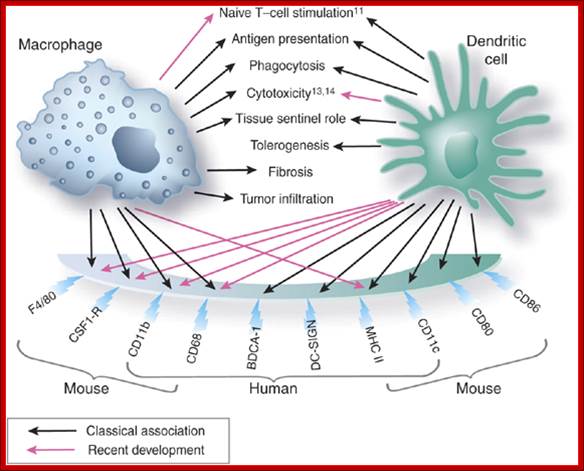
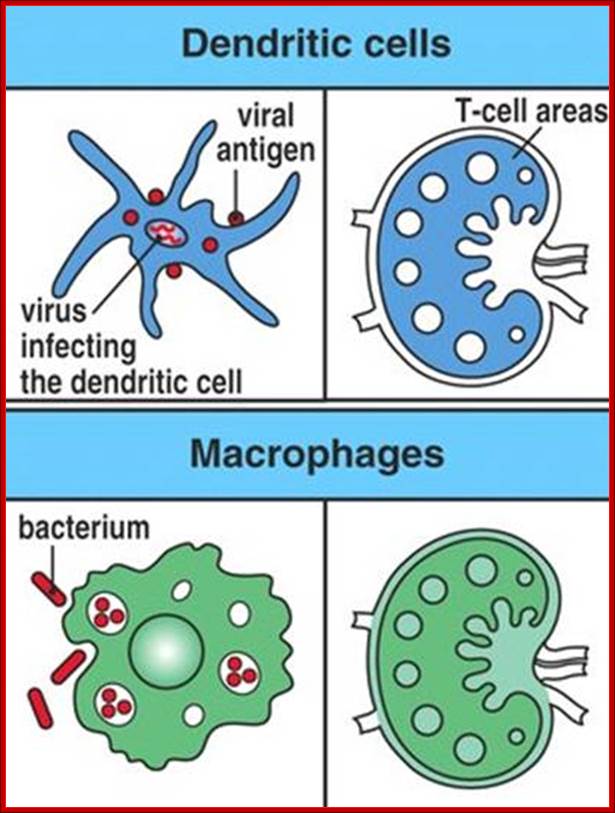
Macrophages and dendritic cells: what is the difference?; Recent advances in the study of both functional characteristics and surface markers of the cells of the mononuclear phagocyte system have led to increasing overlap between what is considered a 'macrophage' and a 'dendritic cell'. Abbreviations: CSF1-R, colony-stimulating factor 1 receptor; BDCA-1, blood dendritic-cell antigen-1; DC-SIGN, dendritic cell-specific ICAM-3-grabbing nonintegrin; MHC II, major histocompatibility complex class II. D Ferenbach and J Hughehttp://www.nature.com/
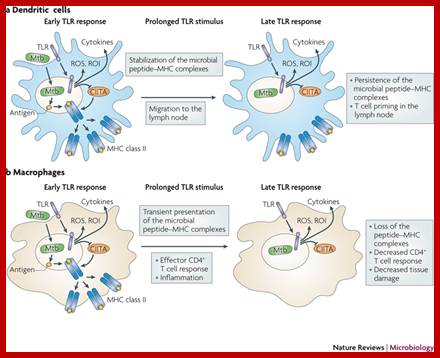
The different responses of macrophages and dendritic cells to Toll-like receptor signalling. Although macrophages and dendritic cells (DCs) both secrete cytokines and produce antimicrobials (such as reactive oxygen species (ROS) and reactive oxygen intermediates (ROI)) in response to Toll-like receptor (TLR) signalling, these cell types differ in how their major histocompatibility complex (MHC) class II antigen presentation function is regulated by TLR signalling. a | In DCs, TLR signalling induces maturation, which involves increased expression of peptide–MHC class II molecule complexes and co-stimulatory molecules. DC maturation also involves migration to lymph nodes, where DCs present antigen to naive T cells, priming the T cell response to pathogens. b | By contrast, macrophages initially show little decrease in MHC class II antigen presentation and then, after prolonged stimulation (approximately 24 hours or more), show inhibition of antigen presentation with decreased expression of MHC class II molecules. This contrast in MHC class II levels results from regulation of the post-translational stability of MHC class II molecules, which is greatly enhanced during DC maturation but is not increased in macrophages upon TLR stimulation. Accordingly, TLR stimulation of DCs by microorganisms results in a final burst of antigen processing and the accumulation of a kinetic cohort of peptide–MHC class II molecule complexes that include microbial peptides; this cohort is expressed for a prolonged period and provides effective stimulation of naive T cells. DCs present antigen in lymph nodes to activate naive T cells, whereas macrophages present antigens to effector T cells at sites of infection, which produces inflammatory responses that can damage host tissues if they are not controlled. This model proposes that macrophages decrease antigen presentation after prolonged TLR stimulation as a means of homeostatic negative-feedback regulation to limit tissue damage from excessive activation of effector T cells. CIITA, MHC class II transactivator; Mtb, Mycobacterium tuberculosis. https://www.researchgate.net/

Dendritic cells (DCs) collect and process antigens for presentation to T cells, but there are many variations on this basic theme. DCs differ in the regulatory signals they transmit, directing T cells to different types of immune response or to tolerance. Although many DC subtypes arise from separate developmental pathways, their development and function are modulated by exogenous factors. Therefore, we must study the dynamics of the DC network in response to microbial invasion. Despite the difficulty of comparing the DC systems of humans and mice, recent work has revealed much common ground.Mouse and human dendrite cells; http://www.nature.com/
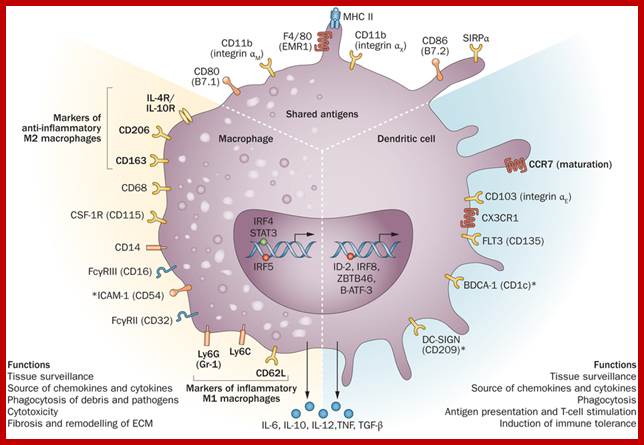
Haematopoietic stem cells (HSCs) differentiate into immature dendritic cells (iDCs) that are recruited to peripheral tissues, where they continuously internalize antigens that can be processed by an endosomal, MHC class-II-restricted pathway. After antigen capture and depending on the nature of the antigen, DCs migrate to the draining lymphoid tissue and mature phenotypically, upregulating the expression of CD40, CD80, CD86, MHC class II molecules and CC-chemokine receptor 7 (CCR7). In the draining lymphoid tissue, they present peptide–MHC class II complexes on the cell surface, interact with antigen-specific lymphocytes and mature functionally, activating T cells, B cells and natural killer (NK) cells and producing pro-inflammatory cytokines, such as interleukin-12 (IL-12) and tumour-necrosis factor (TNF). Renal dendritic cells (DCs) and macrophages represent a constitutive, extensive and contiguous network of innate immune cells that provide sentinel and immune-intelligence activity; they induce and regulate inflammatory responses to freely filtered antigenic material and protect the kidney from infection. Tissue-resident or infiltrating DCs and macrophages are key factors in the initiation and propagation of renal disease, as well as essential contributors to subsequent tissue regeneration, regardless of the aetiological and pathogenetic mechanisms. The identification, and functional and phenotypic distinction of these cell types is complex and incompletely understood, and the same is true of their interplay and relationships with effector and regulatory cells of the adaptive immune system. In this Review, we discuss the common and distinct characteristics of DCs and macrophages, as well as key advances that have identified the renal-specific functions of these important phagocytic, antigen-presenting cells, and their roles in potentiating or mitigating intrinsic kidney disease. We also identify remaining issues that are of priority for further investigation, and highlight the prospects for translational and therapeutic application of the knowledge acquired. Dndritic cells; Holger Hackstein & Angus W. Thomson, http://www.nature.com

Flt3 controls homeostasis of the dendritic cell pool in lymphoid organs.
Macrophage dendritic cell
progenitors (MDPs) are a population of macrophages and DC precursor that are
identified by the expression of the M-CSF receptor (CSF1R, cfms, CD115) and the
absence of lineage markers and that overlap with the more recently described
common DC precursors (CDPs). MDPs give rise to conventional CD11c+ CD8 + and CD11c+ CD8
+ and CD11c+ CD8 -DCs, monocytes and macrophages, and they may also
give rise to plasmacytoid DCs (pDCs)a, although pDC potential was
not observed in the present study. DC progenitors in the bone marrow do not
depend on Flt3 for their homeostatic maintenance in vivo; however,
supraphysiologic levels of Flt3L increase their expansion and their emigration
from the bone marrow. The present study shows that Flt3 is essential to
maintain normal numbers of conventional DCs (cDCs) in lymphoid organs, by
controlling locally the proliferation of DC precursors. In homeostatic
conditions, a small number of DC precursors rapidly transit from bone marrow to
spleen and lymph nodes via the blood as relatively immature cells that do not
express surface MHC II or CD11c. These cells undergo cell divisions within
lymphoid organs, under the control of Flt3L, and differentiate into
conventional CD11c+ CD8
-DCs, monocytes and macrophages, and they may also
give rise to plasmacytoid DCs (pDCs)a, although pDC potential was
not observed in the present study. DC progenitors in the bone marrow do not
depend on Flt3 for their homeostatic maintenance in vivo; however,
supraphysiologic levels of Flt3L increase their expansion and their emigration
from the bone marrow. The present study shows that Flt3 is essential to
maintain normal numbers of conventional DCs (cDCs) in lymphoid organs, by
controlling locally the proliferation of DC precursors. In homeostatic
conditions, a small number of DC precursors rapidly transit from bone marrow to
spleen and lymph nodes via the blood as relatively immature cells that do not
express surface MHC II or CD11c. These cells undergo cell divisions within
lymphoid organs, under the control of Flt3L, and differentiate into
conventional CD11c+ CD8 + and CD11c+ CD8
+ and CD11c+ CD8 - DCs. Lymphotoxin-
- DCs. Lymphotoxin- (LT
(LT 1
1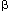 2) has a similar role, restricted to
the homeostasis of the CD8
2) has a similar role, restricted to
the homeostasis of the CD8 - subset of cDCs (14). CMP, common myeloid progenitors; GMP,
granulocyte/macrophage progenitors; HSC, hematopoietic stem cells. Cedric Auffray, Yalin Emre & Frederic Geissmann;
http://www.nature.com/
- subset of cDCs (14). CMP, common myeloid progenitors; GMP,
granulocyte/macrophage progenitors; HSC, hematopoietic stem cells. Cedric Auffray, Yalin Emre & Frederic Geissmann;
http://www.nature.com/
Any cell that can take in the pathogens and process their proteins or CpG sequence nucleic acids is called Antigen Presenting Cells (APC).

Tissue Types and Efficiciency of MHC protein production; https://pt.slideshare.ne
Circulating WBC encounter infected bacteria or viruses and if Abs for such antigens are already present Abs bind all over the surface of the infectious agent and Macrophages opsonize and take them in, then digest, produce small pieces of proteins and present it on its surface with MHC protein, which is recognized by either nave T-cells or fully active T-cells. The kind of T cells recognize the MHC-antigens depends upon the kind of MHCs and the kind of T cell co receptors.
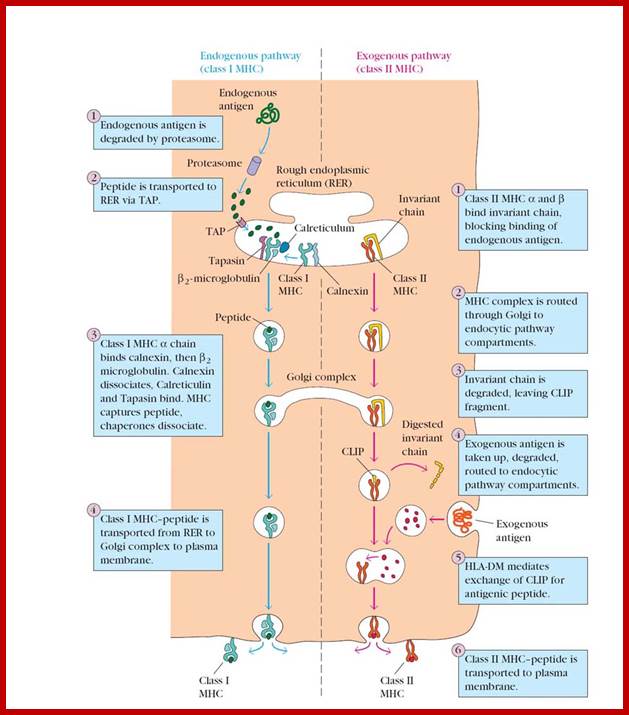
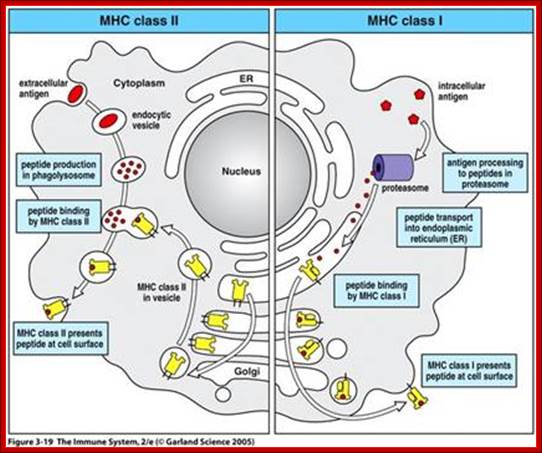
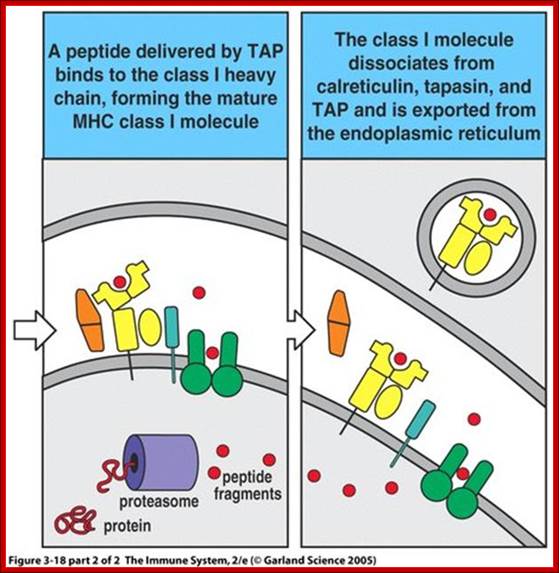
All nucleated cells can present class I associated peptides derive from cytosolic proteins such as viral and tumor antigens to CD8 T cells. Antigen processing means converting native proteins into MHC associated peptides. This process consists of introduction of exogenous protein antigens into APC cells or it involves the synthesis of antigens in cytosol, proteolytic degradation of these proteins into peptides, the binding of peptides to MHC molecular complex and display of the peptide-MHC complexes on the cell surface for recognition by T cells. Antigen processing pathway in APC use basic cellular proteolytic mechanisms that also operates independently of immune system. Both extracellular and intra cellular proteins are sampled by these antigen processing pathways and peptides derived from both normal self proteins and foreign proteins are displayed by MHC, molecules for surveillance by T lymphocytes. For MHC class I associated antigen presentation, cytosolic proteins are proteolytically degraded in proteasomes, generating peptides with features that enable them to bind to class I molecules. These peptides are delivered from cytoplasm to the ER by ATP dependent transporter called TAP. Newly synthesized class I MHC beta2 macroglobulin dimers in ER are attached to the TAP complex and receive peptides transported into ER. Stable complexes of class I MHC molecules with bound peptides move out of ER, through Golgi, to the cell surface.
For MHC class II associated antigen presentation, extracellular proteins are internalized into endosome where they are proteolytically cleaved by lysosomal enzymes that functions at acidic pH. Newly synthesized class MHCII molecules associated with the protein pieces (li) are transported from ER to the golgi membranes where the ‘Ii’, it is a proteolytically cleaved small peptide remnant of Ii called CLIP which is removed from peptide binding cleft of MHC molecule by DM molecules. The peptides that were generated from extracellular proteins then bind to the available cleft of the class MHC II and the trimeric complex (class II MHC alpha and beta chains and peptide) moves to and is displayed on the surface of the cell.
These pathways of MHC’s restricted antigen presentation ensure that most of the body’s cells are screened for the possible presence of foreign antigens, even the proteins produced by viruses in the infected cells. The pathways ensure that proteins from extracellular microbes preferentially generate peptides bound to class II MHC for recognition by CD4 helper T cells, which activate effector mechanisms that eliminate extracellular antigens. Conversely proteins synthesized by intracellular microbes generate peptides bound to class I MHC s for recognition by CD8 CTLs, which function to eradicate cells harboring intracellular infections. The immunogenicity of foreign protein antigens depends on the ability of antigen-processing pathways to generate peptides from these proteins that bind to self MHC molecules.
T cells recognize antigens only in the form of peptides displayed by the products of self MHC genes on the surface of APCs. CD4 helper T lymphocytes recognize antigens associated with class II MHC gene products. Class II MHCs have restricted recognition. Cells with CD8 CTLs recognize antigens in association with class I gene products.
A class of proteins called Major Histocomplex proteins; MHCs are coded for by large loci on chromosome 6 at position p21.3 (from 32,627,656 to base pair 32,634,465). These protein coding genes are also called HLA genes.
The HLA-DQB1 gene is located on the short (p) arm of chromosome 6 at position 21.3. The HLA-B gene is located on the short (p) arm of chromosome 6 at position 21.3. More precisely, the HLA-B gene is located from base pair 31,353,871 to base pair 31,357,211 on chromosome 6.; HLA A and B genes; http://www.nature.com/
MHCs are classed into MHC I and MHC II. The genes for MHC are very polymorphic and consist of 250 genes or more. The role of MHCs was discovered while doing transplantation of tissues. MHC molecules found in the donor tissues react with receiver T-cells and their responses is responsible for rejection of the grafted tissue. MHCs bind peptide antigens and display their antigen on their surfaces for T-cell receptors to recognize them and elicit responses.
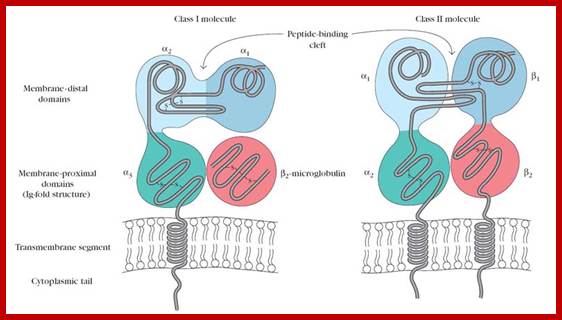
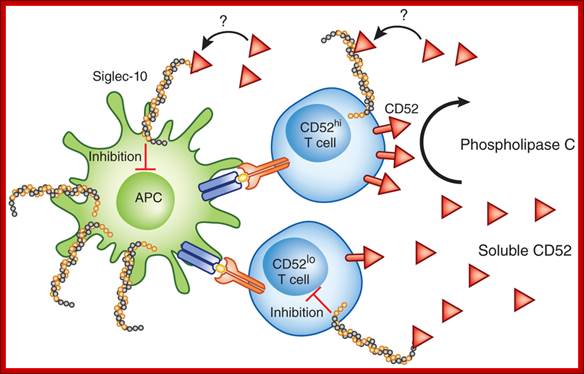
Class I MHCs contain an alpha chain in noncovalently complex with a non polymorphic polypeptide called beta-macroglobulin. But MHC II contains two MHC coded polymorphic alpha and beta chains. Both are structurally more or lea similar and they consist of extracellular peptide binding cleft a non-polymorphic Ig-like protein with exoplasmic and endoplasmic region with an anchoring TM domain. The peptide binding cleft has a helical domain and an eight stranded antiparllel beta pleated sheet floor. The cleft of class I is made up of alpha1 and alpha 2 segments of alpha chains. The Ig like domain of class I and II contain the binding sites for T cell co receptors of CD8 and CD4.
MHC molecules bind only one ones peptide at a time. And all the peptides that bind particularly to MHCs, share common structural motifs. Peptide binding is saturable and the off rate is slow. Once the complex is formed they persist for a long time to be recognized by T cells. The peptide binding cleft of class I can accommodate peptides that are 8-11 a. a long, where as the cleft of class II molecules allow larger peptides of 30 a.a or more. The polymorphic residues of MHC molecules are localized in the peptide binding domain. Some polymorphic ‘MHCs residue recognize and determine the binding specificities for peptides by forming structures called packets, that interact with complementary residues. Other polymorphic residues and few other residues are not involved in binding to MHC molecules, but instead form the structure recognized by T cells. Every MHC molecules has a broad specificity for peptides and can bind multiple peptides and can bind multiple peptides that have common structural features such as anchor residues.
In addition to polymorphic class I and class II genes the MHC also contains genes encoding complement proteins, cytokines a non polymorphic kinds of proteins called class I like molecules and several protein involve in antigen processing.
Class I MHCs are expressed in nucleated cells. But MHC class II are expressed mainly antigen presenting cell types such dendrite cells, macrophages and B lymphocytes and few other cell types including endothelial cells and thymic epithelial cells. The expression MHC genes products are enhanced by inflammatory and immune stimuli particularly cytokines like IFN gamma which stimulate transcription MHC class of genes.
Any cell that can take in the pathogens and process their proteins or CpG sequence nucleic acids is called Antigen Presenting Cells (APC). file:///C:/Users
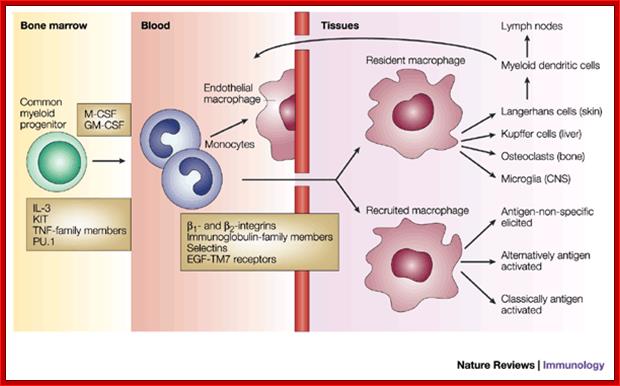
Regulation unmasked by activation;
CD4+ T cells are activated by antigen presented by antigen-presenting cells (APC) such as dendritic cells. Phospholipase C cleaves CD52 from CD52hi CD4+T cells. Soluble CD52 binds to Siglec-10 expressed on activated T cells and inhibits the T cell response. Given the high expression of Siglec-10 on cells of the innate immune system, it seems likely that CD52 may also act via such cells. Additionally, Siglec-10 expression on activated T cells raises the possibility that CD52 acts in an autocrine manner, although the present paper by Bandala-Sanchez et al.1 does not directly address either of those possibilities. Mike Clark & Anne Cooke http://www.nature.com/;
Human MHC class I and II are also called human leukocyte antigen (HLA)
The most intensely studied HLA genes are the nine so-called classical MHC genes: HLA-A, HLA-B, HLA-C, HLA-DPA1, HLA-DPB1, HLA-DQA1, HLA-DQB1, HLA-DRA, and HLA-DRB1. In humans, the MHC is divided into three regions: classes I, II, and III. The A, B, C, E, F, and G genes belong to MHC class I, whereas the six D genes belong to class II.
MHC genes are expressed in codominant fashion.[5] This means that the alleles (variants) inherited from both progenitors are expressed in equivalent way:
- As there are 3 Class-I genes, named in humans HLA-A, HLA-B and HLA-C, and as each person inherits a set of genes from each progenitor, that means that any cell in an individual can express 6 different types of MHC-I molecules (see figure).
- In the Class-II locus, each person inherits a couple of genes HLA-DP (DPA1 and DPA2, which encode α and β chains), a couple of genes HLA-DQ (DQA1 and DQA2, for α and β chains), one gene HLA-DRα (DRA1) and one or two genes HLA-DRβ (DRB1 and DRB3, -4 o -5). That means that one heterozygous individual can inherit 6 or 8 Class-II alleles, three or four from each progenitor.
- The MHC genes are highly polymorphic; this means that there are many different alleles in the different individuals inside a population. The polymorphism is so high that in a mixed population (non-endogamic) there are not two individuals with exactly the same set of MHC genes and molecules, with the exception of identical twins.
- This imposes a very specific link between the MHC molecule and the peptide, and it implies that each MHC variant will be able to bind specifically only those peptides that is able to properly enter in the cleft of the MHC molecule, which is variable for each allele. In this way, the MHC molecules have a broad specificity, because they can bind many, but not all types of possible peptides
- MHCs exhibit polymorphism.
Human MHC class I and II are also called human leukocyte antigen (HLA); http://en.wikipedia.org/


Class I and class 2 respectively; http://en.wikipedia.org/
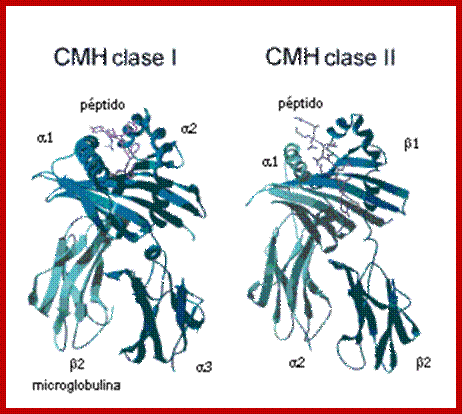
Class I
MHC I occurs as an α chain composed of three domains—α1, α2, α3. The α1 rests upon a unit of the non-MHC molecule β2 microglobulin (encoded on human chromosome 15). The α3 subunit is trans-membrane, anchoring the MHC class I molecule to the cell membrane. The peptide being presented is held by the floor of the peptide-binding groove, in the central region of the α1/α2 heterodimer (a molecule composed of two nonidentical subunits). The genetically encoded and expressed sequence of amino acids, the sequence of residues, of the peptide-binding groove's floor determines which particular peptide residues it binds.[7]
Class II;
MHC class two is formed of two chains, α and β, each having two domains—α1 and α2 and β1 and β2—each chain having a transmembrane domain, α2 and β2, respectively, anchoring the MHC class II molecule to the cell membrane.[8] The peptide-binding groove is formed of the heterodimer of α1 and β1.
MHC class II molecules in humans have five to six Isotypes. Classic molecules present peptides to CD4+ lymphocytes. Nonclassic molecules, accessories, with intracellular functions, are not exposed on cell membranes, but in internal membranes in lysosomes, normally loading the antigenic peptides onto classic MHC class II molecules.
Class III;
Class III molecules have physiologic roles unlike classes I and class II, but are encoded between them in the short arm of human chromosome 6. Class III molecules include several secreted proteins with immune functions: components of the complement system (such as C2, C4, and B factor), cytokines (such as TNF-α, LTA, LTB), and heat shock proteins (hsp). From Wikipedia
http://www.biologia.edu.ar;https://www.pinterest.com
Class I
MHC Class Proteins: MHC proteins show likeliness of immunoglobulin proteins.
MHC I occurs as an α chain composed of three domains—α1, α2, α3. The α1 rests upon a unit of the non-MHC molecule β2 microglobulin (encoded on human chromosome 15). The α3 subunit is transmembrane, anchoring the MHC class I molecule to the cell membrane. The peptide being presented is held by the floor of the peptide-binding groove, in the central region of the α1/α2 heterodimer (a molecule composed of two nonidentical subunits). The genetically encoded and expressed sequence of amino acids, the sequence of residues, of the peptide-binding groove's floor determines which particular peptide residues it binds.
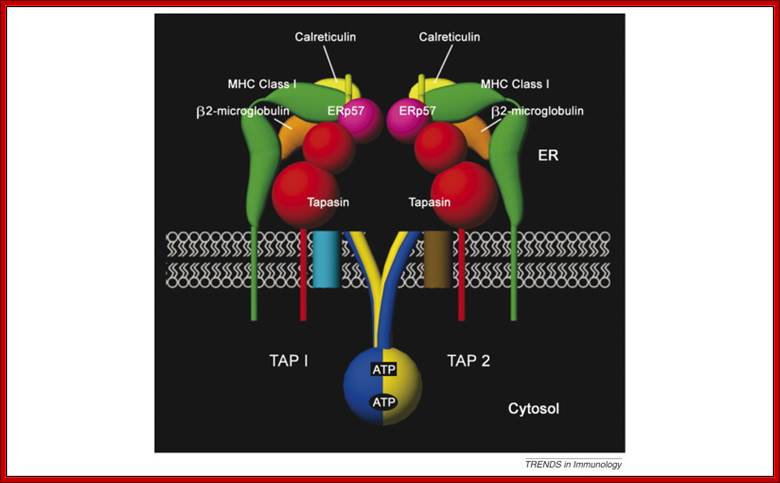
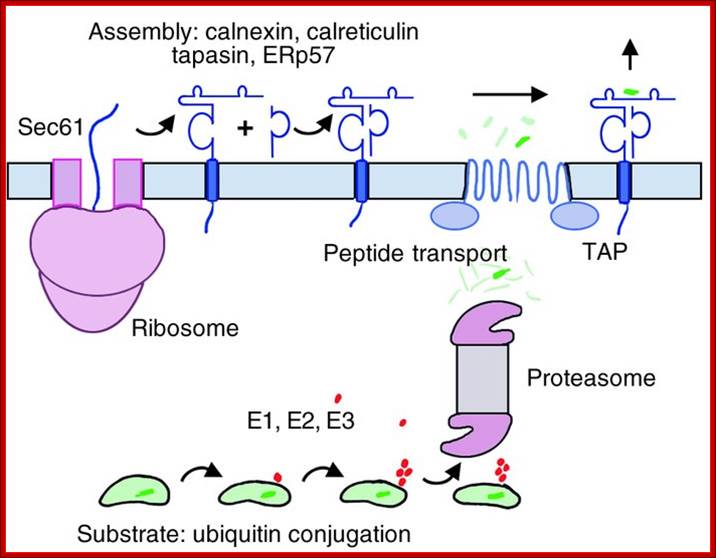
The assembly of major histocompatibility complex (MHC) class I molecules with peptides is orchestrated by several assembly factors including the transporter associated with antigen processing (TAP) and tapasin, the endoplasmic reticulum (ER) oxido-reductases ERp57 and protein disulfide isomerase (PDI), the lectin chaperones calnexin and calreticulin, and the ER amino peptidase (ERAAP). Typically, MHC class I molecules present endogenous antigens to cytotoxic T lymphocytes (CTLs). However, the initiation of CD8+ T-cell responses against many pathogens and tumors also requires the presentation of exogenous antigens by MHC class I molecules. We discuss recent developments relating to interactions and mechanisms of function of the various assembly factors and pathways by which exogenous antigens access MHC class I molecules. Malini Raghavan, Natasha Del Cid
Syed Monem Rizvi ,Larry Robert Peters’ ;http://www.cell.com
https://www.studyblue.com

Structure of MHC Class I (A) and class II proteins (B). The two globular domains furthest from the plasma membrane that form the peptide binding region (PBR) are shaded in blue. The two Ig-like domains, including the β 2 -microglobulin of class II, are shaded in grey. Figure adapted from Alberts et al. 2008: https://www.researchgate.net
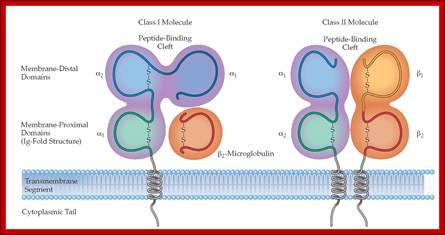
Adaptive immunity: MHC molecules are of two structural types with very similar peptide-binding sites on the membrane-distal surface. (a) MHC class I molecules consist of heavy chains made up of three polypeptide domains (aj, a2, a3) and a noncovalently associated light chain, ^-microglobulin. (b) MHC class II molecules are heterodimers of a and p chains with a very similar overall structure and peptide-binding surface. http://what-when-how.com/
MHC class II protein molecule
Classical MHC molecules present epitopes to the TCRs of CD8+ T lymphocytes. Nonclassical molecules (MHC class IB) exhibit limited polymorphism, expression patterns, and presented antigens; this group is subdivided into a group encoded within MHC loci (e.g., HLA-E, -F, -G) as well as those not (e.g., stress ligands such as ULBPs, Rae1, H60); the antigen/ligand for many of these molecules remain unknown, but they can interact with both CD8+ T cells, NKT cells, and NK cells.
MHC class II proteins
MHC class two is formed of two chains, α and β, each having two domains—α1 and α2 and β1 and β2—each chain having a transmembrane domain, α2 and β2, respectively, anchoring the MHC class II molecule to the cell membrane. The peptide-binding groove is formed of the heterodimer of α1 and β1.

http://pathmicro.med.sc.edu/
MHC class II molecules in humans have five to six isotypes. Classic molecules present peptides to CD4+ lymphocytes. Nonclassic molecules, accessories, with intracellular functions, are not exposed on cell membranes, but in internal membranes in lysosomes, normally loading the antigenic peptides onto classic MHC class II molecules.
Class III
Class III molecules have physiologic roles unlike classes I and class II, but are encoded between them in the short arm of human chromosome 6. Class III molecules include several secreted proteins with immune functions: components of the complement system (such as C2, C4, and B factor), cytokines (such as TNF-α, LTA, LTB), and heat shock proteins (hsp). Class III function very differently from class I and class II, but its locus occurs between the other two classes—on chromosome 6 in humans—and are frequently discussed together. Cactin targets the MHC class III protein IkappaB-like (IkappaBL) and inhibits NF-kappaB and interferon-regulatory factor signaling pathways. Class III MHC genes include the complement system (i.e. C2, C4a, C4b, Bf). Complement proteins help to activate and maintain the inflammatory process of an immune response (eNotes). In addition to components of the complement system MHC III genes code inflammatory cytokines, tumor necrosis factor a and 0 (TNF a and P), two heat shock proteins (HSP) etc. They are not membrane proteins and have no role in Ag presentation. MHC Class III molecules are not structurally related to class I and class II molecules but are important in immune response.
Assembly of MHC Proteins in ER:
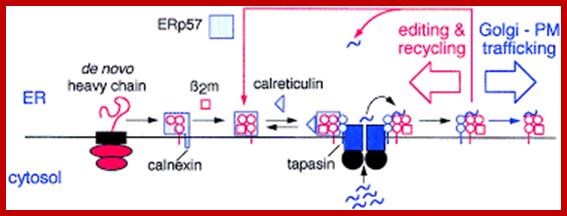
Within the ER, newly synthesized H chains (green) associate with a membrane-bound molecular chaperone termed calnexin (orange) and another protein ERp57 (purple). The calnexin-H chain complex then binds to the beta2m subunit (green). At this point, calnexin may remain or it may be replaced by its soluble homolog calreticulin. Yet another protein, Bap31 (blue), binds at this stage. Subsequent association with the TAP transporter (orange) occurs in an interaction that is bridged by another protein termed tapasin (pink). This multi-subunit complex consisting of calnexin (or calreticulin), H chain, beta2m, tapasin, TAP, ERp57 and Bap31 is known as the "peptide-loading complex" or PLC. Following peptide binding to class I, the peptide loading complex dissociates and the fully assembled class I molecule is exported to the cell surface. Once at the cell surface, peptide-class I complexes are scrutinized by T cell receptors on circulating cytotoxic T cells. If the T cell receptor binds with sufficiently high affinity, the infected cell will be killed.; http://physrev.physiology.org
Class I complex synthesis and assembly. MHC class I heavy chains are cotranslationally inserted into the ER. Proper folding, association with the β2m light chain, and peptide loading are assisted by chaperones such as calnexin, calreticulin, tapasin, and ERp57. E1, E2, and E3 ubiquitin-conjugating enzymes tag proteins in the cytosol for destruction by the proteasome. Peptides are pumped into the ER by the TAP transporters. Properly folded, peptide-loaded MHC class I complexes progress through the secretory pathway for display on the cell surface. TAP, transporter associated with antigen processing; http://www.jci.org/
How human cytomegalovirus evades the antigen presentation process by causing the premature degradation of class I molecules through a process termed ER-associated degradation or ERAD. ERAD is a normal part of the ER’s quality control system (see ERAD section of website www.grkraj.org) that functions to detect and destroy misfolded proteins. Intriguingly, this process has been commandeered by the virus (Figure below).

Figure above: Rapid ERAD disposal of MHC class I molecules by cytomegalovirus US2 and US11 proteins. US2 and 11 bind to newly synthesized class I heavy chains (panel B) and direct the heavy chains to ERAD disposal pathways. There are several incompletely defined ERAD pathways; the one illustrated utilizes the Hrd1-SEL1L complex (panel A). The molecular chaperones Cnx, Crt, BiP and Grp94 play a role in the initial recognition of misfolded proteins. Additional proteins such as EDEM and Os9 are thought to participate in substrate recognition and also to target substrates to the retrotranslocation machinery. Retro translocation to the cytosol occurs via the Hrd1-Sel1 complex. In mammals, this complex likely includes: Hrd1 (an E3 ubiquitin ligase/potential retrotranslocon), Sel1 (Hrd1 interaction partner), Derlin-1, -2 or -3 (potential retrotranslocons), UBC7 (ubiquitin-conjugating enzyme) and the p97-Ufd1-Npl4 AAA-ATPase complex (powers retrotranslocation). During or following retro translocation, substrates are ubiquitinated, N-linked oligosaccharides are removed and degradation by the proteasome ensues. Williams Lab; http://biochem17.med.utoronto.ca/

A proposed model for the degradation of
MHC heavy chains from the ER; Class I MHC heavy chain molecules are inserted
into the ER where the N-linked oligosaccharide is transferred from a
dolicholpyrophosphate carrier onto the Asn-X-Ser/Thr acceptor sequence in the
nascent chain (Silberstein and Gilmore, 1996).
The N-linked oligosaccharide carries three glucose residues that are removed
sequentially by ER glucosidases I and II (GI/GII). The concerted action of
glucosidase II and UDP-glucose: UGT constitutes a cycle that deglucosylates and
reglucosylates the oligosaccharide on the folding polypeptide chain. Calnexin
and calreticulin bind to the monoglucosylated form of the oligosaccharide and
assist in folding of the polypeptide. Binding of ![]() 2m and a peptide allows the heavy chain to exit to the Golgi where
the high-mannose type N-linked glycan is converted to a complex type glycan
before the fully assembled complex reaches the cell surface. Proteins that fail
to fold properly or do not assemble with their appropriate binding partners are
retained in the ER and become a substrate of ER mannosidase I (not shown) after
a certain lag time, as do properly folded glycoproteins, yielding a Man8
structure. A Man8 structure on a misfolded protein signals that the polypeptide
has resided in the ER for some time without acquiring its native structure.
Such proteins are extracted from the ER and become ubiquitylated by
ubiquitylating enzymes that may reside at the cytosolic face of the ER
membrane. A recently discovered E3 ubiquitin ligase, Fbx2, recognizes N-linked
glycans in the cytosol and may trigger selective ubiquitylation of
glycoproteins upon their arrival in the cytosol (Yoshida et al., 2002).
The 26S proteasome degrades the glycoprotein after its deglycosylation by N-glycanase.
The deglycosylation results in the conversion of the asparagine that carried
the N-linked glycan to an aspartate. It has not yet been proven whether
cytosolic oligosaccharides carry a terminal Glc residue; Christian Hirsch, Daniël Blom, Hidde L. Ploeghhttp://emboj.embopress.org/
2m and a peptide allows the heavy chain to exit to the Golgi where
the high-mannose type N-linked glycan is converted to a complex type glycan
before the fully assembled complex reaches the cell surface. Proteins that fail
to fold properly or do not assemble with their appropriate binding partners are
retained in the ER and become a substrate of ER mannosidase I (not shown) after
a certain lag time, as do properly folded glycoproteins, yielding a Man8
structure. A Man8 structure on a misfolded protein signals that the polypeptide
has resided in the ER for some time without acquiring its native structure.
Such proteins are extracted from the ER and become ubiquitylated by
ubiquitylating enzymes that may reside at the cytosolic face of the ER
membrane. A recently discovered E3 ubiquitin ligase, Fbx2, recognizes N-linked
glycans in the cytosol and may trigger selective ubiquitylation of
glycoproteins upon their arrival in the cytosol (Yoshida et al., 2002).
The 26S proteasome degrades the glycoprotein after its deglycosylation by N-glycanase.
The deglycosylation results in the conversion of the asparagine that carried
the N-linked glycan to an aspartate. It has not yet been proven whether
cytosolic oligosaccharides carry a terminal Glc residue; Christian Hirsch, Daniël Blom, Hidde L. Ploeghhttp://emboj.embopress.org/
Transcription of MHC genes: Peter J. van en Elsen, Marja C.J.A. van Eggermond and Rutger J. Wierda
This chapter describes recent advances in our understanding how epigenetic events control immune functions with emphasis on transcriptional regulation of major histocompatibility complex Class I (MHC‑I) and Class II (MHC‑II) genes. MHC‑I and MHC‑II molecules play an essential role in the adaptive immune response by virtue of their ability to present peptides, respectively to CD8+ and CD4+ T cells. Central to the onset of an adequate immune response to pathogens is the presentation of pathogen‑derived peptides in the context of MHC‑II molecules by antigen presenting cells (APCs) to CD4+ T cells of the immune system. In particular dendritic cells are highly specialized APCs that are capable to activate naïve T cells.
Given their central role in adaptive immunity, MHC‑I and MHC‑II genes are regulated in a tight fashion at the transcriptional level to meet with local requirements of an effective antigen‑specific immune response. In these regulatory processes the MHC2TA encoded Class II trans activator (CIITA) plays a crucial role. CIITA is essential for transcriptional activation of all MHC‑II genes, whereas it plays an ancillary function in the transcriptional control of MHC‑I genes. The focus of this chapter therefore will be on the transcription factors that interact with conserved cis‑acting promoter elements and epigenetic mechanisms that modulate cell type‑specific regulation of MHC‑I, MHC‑II and MHC2TA genes. Furthermore, we will also briefly discuss how genetic and epigenetic mechanisms contribute to T helper cell differentiation.
The MHC class II enhanceosome; Critical DNA sequences for transcriptional regulation of the MHC class II genes are represented by the X1, X2 and Y boxes in the proximal promoter. Similar X and Y boxes have been found upstream and may represent a locus control region (LCR). Seven basal transcription factors bind these cis elements—the trimeric (Tri) RFX complex, cAMP-response element binding protein (Creb) and the trimeric (ABC) NFY complex. These transcription factors interact with the basal transcription machinery (TBP, TAF-1 and PolII), the ATP-dependent chromatin remodeling component BRG-1, the histone methyltransferase CARM-1 and several other coactivators as shown in the figure. The MHC class II transactivator, CIITA, is considered the ‘master regulator’ and coordinates the interactions at this enhanceosome. Six of the enhanceosome components have HAT activity—CBP, p300, PCAF, SRC-1, TAF-1 and CIITA.
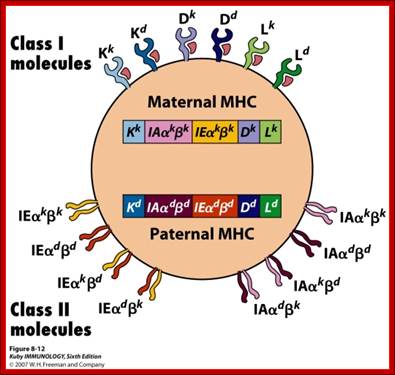
Maternal and paternal contribution to MHC class of proteins; http://adrien.six.online.fr/http://users.utu.fi/
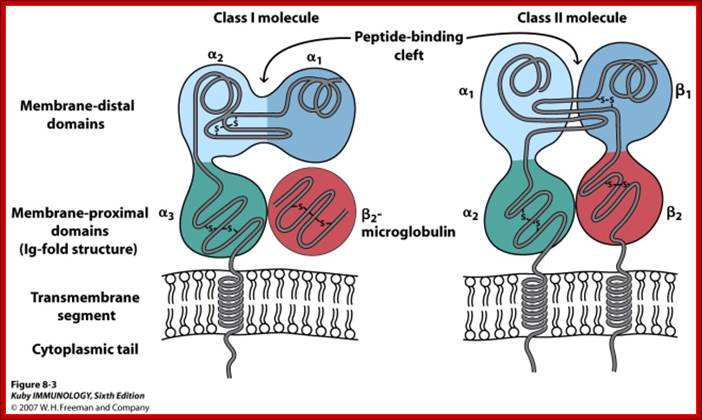
MHC II alpha and beta mol wt. 33 and 28Kd (230 n 240 a.a);MHCI beta2 microglobulin-12KD, alpha (3domains -45kd Fig-repeat

|
Table . Characteristics of the antigen processing pathways |
||
|
Characteristic |
MHC-I pathway |
MHC-II pathway |
|
Composition of the stable peptide-MHC complex |
Polymorphic chain α and β2 microglobulin, peptide bound to α chain |
Polymorphic chains α and β, peptide binds to both |
|
Types of antigen presenting cells (APC) |
All nucleated cells |
Dendritic cells, mononuclear phagocytes, B lymphocytes, some endothelial cells, epithelium of thymus |
|
T lymphocytes able to respond |
Cytotoxic T lymphocytes (CD8+) |
Helper T lymphocytes (CD4+) |
|
Origin of antigenic proteins |
cytosolic proteins (mostly synthetized by the cell; may also enter from the extracellular medium via phagosomes) |
Proteins present in endosomes or lysosomes (mostly internalized from extracellular medium) |
|
Enzymes responsible for peptide generation |
Cytosolic proteasome |
Proteases from endosomes and lysosomes (for instance, cathepsin) |
|
Location of loading the peptide on the MHC molecule |
Specialized vesicular compartment |
|
|
Molecules implicated in transporting the peptides and loading them on the MHC molecules |
TAP (transporter associated with antigen processing) |
DM, invariant chain |
MHC Genes:
In humans the MHC region occurs on chromosome 6, between the flanking genetic markers MOG and COL11A2, and contains 140 genes spanning 3.6 mega base pairs (3.6 Mb or 3 600 000 bits).[2] About half have known immune functions
Class III function very differently from class I and class II, but its locus occurs between the other two classes—on chromosome 6 in humans—and are frequently discussed together.
Induction of MHC protein synthesis:
Most of the MHC lass proteins are induced their gene expression by P53 as antitumor response. Major histocompatibility complex (MHC) class II genes are expressed constitutively in only a few cell types, but they can be induced in the majority of them, in particular by interferon-gamma (IFN-gamma). The MHC class II trans activator gene CIITA is defective in a form of primary MHC class II deficiency. Here it is shown that CIITA expression is controlled and induced by IFN-gamma. A functional CIITA gene is necessary for class II induction, and transfection of CIITA is sufficient to activate expression of MHC class II genes in class II-negative cells in the absence of IFN-gamma. CIITA is therefore a general regulator of both inducible and constitutive MHC class II expression. Untreated control cultures did not express MHC class II antigens. However, following exposure to either recombinant bovine IFN-gamma or TNF-alpha (rBoIFN-gamma, rBoTNF-alpha) MHC class II (Ia) antigen expression was induced on these nonlymphoid cell types. rBoIFN-alpha I 1 did not induce class II antigens, but suppressed their induction by rBoIFN-gamma and TNF-alpha. Here, we show that bacteria induce de novo synthesis of both major histocompatibility complex (MHC) class I and II molecules in a mouse dendritic cell culture system. The neo-biosynthesis of MHC class I molecules is delayed as compared with that of MHC class II. Furthermore, bacteria stabilize MHC class I molecules by a 3-fold increase of their half-life. This has important consequences for the capacity of dendritic cells to present bacterial antigens in the draining lymph nodes.
Table 1
|
Encoding |
|
(1) peptide-binding proteins, which select short sequences of amino acids for antigen presentation, as well as (2) molecules aiding antigen-processing |
|
(1) peptide-binding proteins and (2) proteins assisting antigen loading onto MHC class II's peptide-binding |
|
Expression |
|
One chain, called α, whose ligands are the CD8 receptor—borne notably by cytotoxic T cells and inhibitory receptors borne by NK cells. |
|
Two chains, called α & β, whose ligands are the CD4 receptors borne by |
|
Various Antigens can be-
|
- proteins
- polysaccharides,
- Glyco-or proteo glycans,
- Conjugates of lipids with-
- proteins (lipoproteins) and
- polysaccharides (glycolipids).
- All are macromolecules
Inhaled macromolecules, ingested macromolecules, vaccine injected under the skin,Even antigens from viruses and cancer cells can develop inside the cell.
Exogenous antigens – processed and presented by MHC II class proteins.
Antigen-presenting cells:
- Engulf the antigen by endocytosis.
- The endosome fuses with a lysosome where the antigen is degraded into fragments (e.g. short peptides).
- These antigenic peptides are then displayed at the surface of the cell nestled within a class II histocompatibility molecule.
- Here they may be recognized by CD4+ T cells-called helper T cells (Th)
- Macrophages, Dendrite cells and B cells.
- MHCII two TM proteins, third found in the groove. All are found in ER; a protein called Li finds the groove and stays till the antigen binds
- Antigen bind to their groove, This trimoecular complex moves to GB and then to into lysosomes,
- Endocytosed antigens enter into endosomes and fuse with lysosomes,
- The digested antigens , the Li chains are digested, this frees the groove for the binding of antigenic fragment, This moves PM;
- This MHC II binds to T cell with CD4; Cross-presentation is the transferring of extracellular antigens like bacteria, some tumor antigens, and antigens in cells infected by viruses into the class I pathway for stimulation of CD8+ cytotoxic T cells (CTL).
- A viral antigen presented on cell surfaces (other than dendrite and macrophages) is the target for cytotoxic T cells (CTLs).
- Lack of costimulants make CTLs fail to develop clones
- Dead cells go through endocytosis, protein degradation, TAP transfer them into ER and to MHC1. Cyotoxic CD8 cells bind and expand clonally lysosomes and present on MHC1
- Self-proteins develop autoimmunity, by deleting CD4 cells.
- Proteins synthesized by viruses can induce CD4 and alsoCD8 cells.
(Dendritic cells can also present intact antigen directly to B cells. In this case, the engulfed antigen is not degraded in lysosomes but is returned to the cell surface for presentation to B cells bearing BCRs of the appropriate specificity.)
Endogenous Antigens- Processed and Presented by MHC I class Proteins:
Antigens that are generated within a cell (e.g., viral proteins in any infected cell) are
- Degraded into fragments (e.g., peptides) within the cell and displayed at the surface of the cell nestled within a class I histocompatibility molecule.
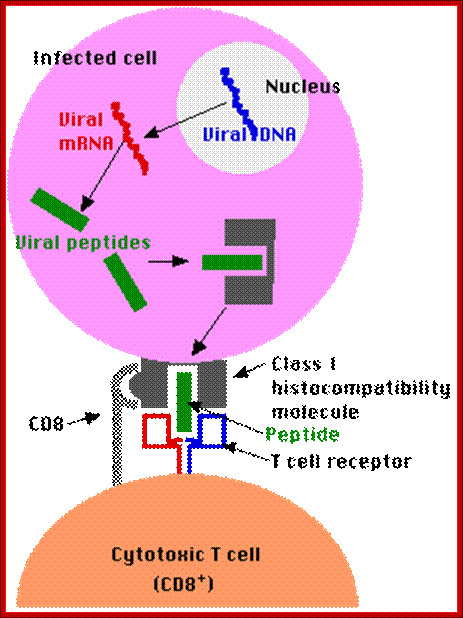
http://users.rcn.com/jkimball.ma.ultranet
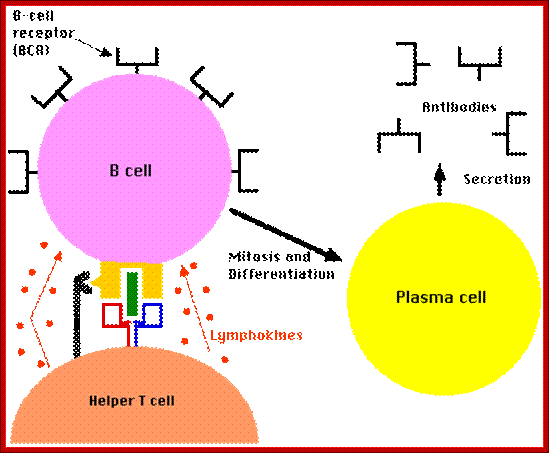
http://users.rcn.com/jkimball.ma.ultranet
Here they may be recognized by CD8 cells. http://www.cs.stedwards.edu/
- Most CD8+ T cells are cytotoxic.
- They have the machinery to destroy the infected cell (often before it is able to release a fresh crop of viruses to spread the infection)
- Peptide production - LMP2/LMP7
- Peptide transporters - TAP1/TAP2
- Antigen presentation
- Helper molecules - Tapasin
- The antigenic peptide binds to beta-2 microglobulin,
- Viral proteins-degraded, proteins are picked up by TAPs in ER, TAPs are ABC group of transporters, and then Proteins are pumped into ER.
- There they bind to TM and beta 2-microglobulns, This complex moves to GB, then to PM;
- This complex binds to TCR, CD8 binds to MHC 1
Note-CTLs which recognize self-peptides (i.e. peptides produced by the normal host body as opposed to foreign or cancerous cells) are removed in the thymus or tolerized after their release from the thymus.
B lymphocytes can act as APCs:
They bind to antigens and also act as APCs. They bind to viruses in the surrounding lymph, or by APCs (macrophages and dendrite cells).
They endocytose Viruses via BCRs, antigen is processed and loaded on to MHC II then expressed on PM. CD4 cells recognize, bind and stimulated to release lymphokines, The CD4 cells are called Helper T cell Th. B cells activated and start dividing, they generate plasma cells and memory cells, they produce only one type of Igs for the said antigen.
Dendrite Cells can present antigens directly to B cells and activate them:
Dr Qi and Ron Germain they captured images of B cells being activated by dendritic cells in a live mouse. They employed a trick that makes B cells light up in a distinct color when they detect antigens—something not previously done in a living mouse—which helped them spot exactly when and where those B cells were being activated.
They observed B cells entering the lymph nodes and becoming activated after interacting with dendritic cells bearing the correct antigens. All this happened before the B cells could enter the follicles. What the microscopic videos show, says Dr. Qi, is that besides their more familiar role in activating helper T cells, dendritic cells carrying antigen to the lymph nodes can present their antigen to B cells and activate them. Drs. Qi and Germain think that this may be an important solution for how our B cells could produce antibodies to big pieces of antigens. Furthermore, by presenting antigen to B cells and helper T cells at the same time in nearby locations, dendritic cells may also serve to foster meetings of T cells and B cells that recognize the same antigen. To scientists like Drs. Qi and Germain, this is a novel aspect of dendritic cell biology that may one day open up new ways of boosting antibody responses. Even macrophages directly activate B cells by presenting the antigens. The SCS macrophages, shown in (B) , have been the particular focus of a number of recent studies. These macrophages accumulate larger antigens potentially through a variety of cell surface receptors according to the nature of the antigen itself. Accumulated antigen might either move along the cell surface or enter intracellular vesicles and be recycled intact to the cell surface for presentation to cognate B cells through the B-cell receptor (BCR), or to non-cognate B cells through complement receptors. But How?
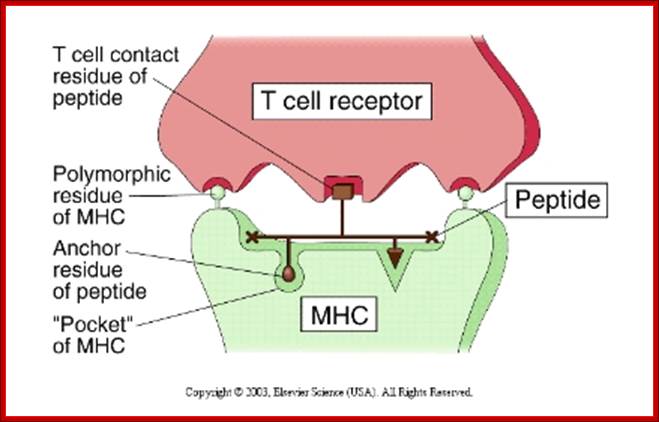
This illustration shows a T-cell receptor (TCR) on the surface of a T-lymphocyte recognizing two polymorphic residues of a MHC molecule and one residue of its bound peptide epitope. http://faculty.ccbcmd.edu
Structual basis :
· The "floor" of the MHC groove has "pockets".
- The peptides that bind have "Anchor"AA residues.
- Peptides can be eluted out of the cleft and studied structurally.
Opsonization and antigen processing and presentation- Just some figures

https://www.studyblue.com
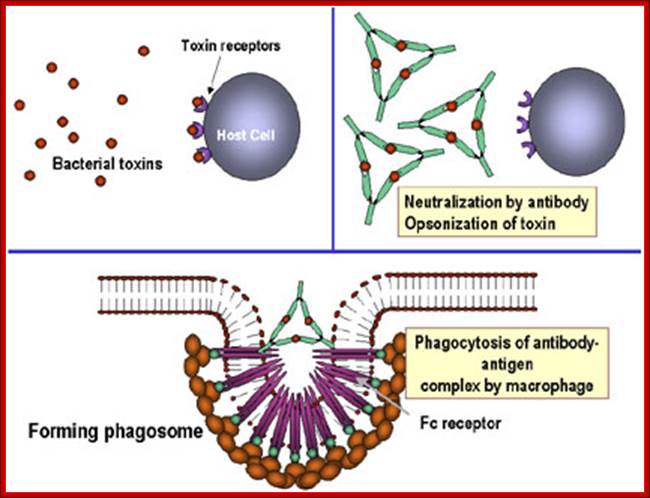
Antibodies binding to and neutralizing bacterial toxin proteins and preventing it from interacting with host cells and causing pathology; Unbound toxin can react with receptors on the host cell, whereas the toxin: antibody complex cannot. Antibodies also neutralize complete virus particles and bacterial cells by binding to them and inactivating them. The antigen: antibody complex is eventually scavenged and degraded by macrophages. Antibodies coating an antigen render it recognizable as foreign by phagocytes (macrophages and polymorphonuclear leukocytes), which then ingest and destroy it; this is called opsonization. http://pathmicro.med.sc.edu/
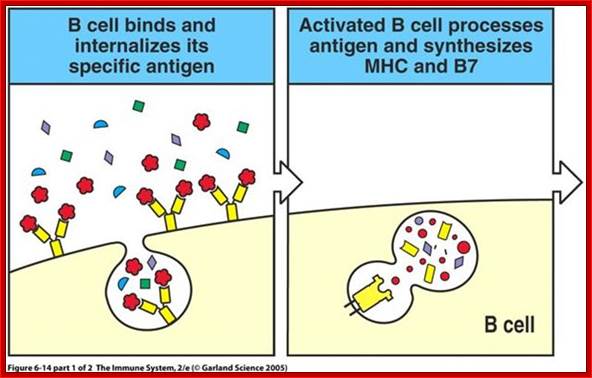
When B cells bind to invading or circulating specific antigens, AB-antigen complex is endocytosed and they same is processed and loaded on to the MHC proteins and transported to the surface of B cells; here B cells act as APCs.
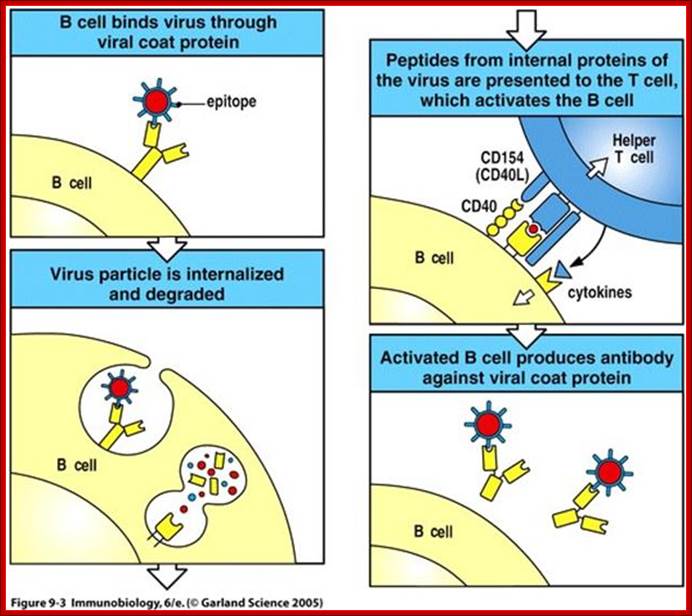
When a B cell presents engulfed antigen on MHC on its cell surface, it can interact with T cells having complementarity of binding surface with its receptor proteins. T cells can bind only protein epitopes and not other poly saccharides, liposaccharides as general Abs and B cell do. http://slideplayer.com
If the MHC class II carry the antigens, it will be recognized by T-cells, can ne an helper cell or otherwise, only if the receptors contain CD4 coregulator, otherwise not. If the MHC 1 carries the antigen it will be recognized by T cells that have CD28 as co receptor, otherwise no recognition. Very often APC also provide co-stimulating protein along with specific MHCs, makes the T cell active provided it also contains Co-receptor CD28; otherwise the T cell commits suicide or PCD.
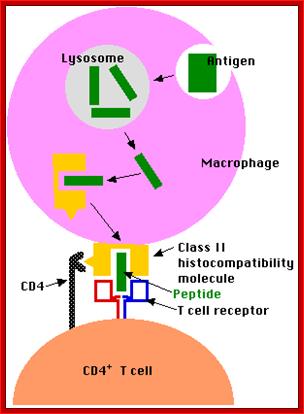
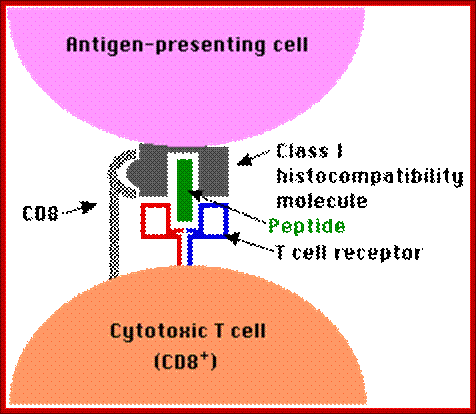
Antigen presenting cell interacting with CD4 T cell and Cytotoxic T CD8 cell; Kimball’s pages; http://www.biology-pages.info